Synthesis and distribution of CARDS toxin during Mycoplasma pneumoniae infection in a murine model
- PMID: 21957154
- PMCID: PMC3222103
- DOI: 10.1093/infdis/jir557
Synthesis and distribution of CARDS toxin during Mycoplasma pneumoniae infection in a murine model
Abstract
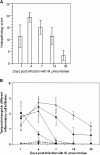
Mice were infected with Mycoplasma pneumoniae and monitored for the synthesis and distribution of the unique adenosine diphosphate-ribosylating and vacuolating Community Acquired Respiratory Distress Syndrome (CARDS) toxin in bronchiolar lavage fluid (BALF) and lung. We noted direct relationships between the concentration of CARDS toxin and numbers of mycoplasma genomes in BALF and the degree of histologic pulmonary inflammation. Immunostaining of lungs revealed extensive colonization by mycoplasmas, including the detection of CARDS toxin in the corresponding inflamed airways. Lung lesion scores were higher during the early stages of infection, decreased gradually by day 14 postinfection, and reached substantially lower values at day 35. Infected mouse immunoglobulin (Ig) M and IgG titers were positive for CARDS toxin as well as for the major adhesin P1 of M. pneumoniae. These data reinforce the proposed pathogenic role of CARDS toxin in M. pneumoniae-mediated pathologies.
Figures






References
-
- Baseman JB, Reddy SP, Dallo SF. Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections. Am J Respir Crit Care Med. 1996;154:S137–44. - PubMed
-
- Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. - PubMed

