A biochemical fluorometric method for assessing the oxidative properties of HDL
- PMID: 21957198
- PMCID: PMC3220300
- DOI: 10.1194/jlr.D018937
A biochemical fluorometric method for assessing the oxidative properties of HDL
Abstract
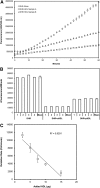
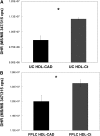
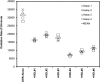
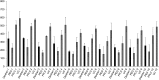
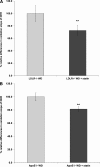
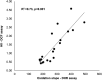
Most current assays of HDL functional properties are cell-based. We have developed a fluorometric biochemical assay based on the oxidation of dihydrorhodamine 123 (DHR) by HDL. This cell-free assay assesses the intrinsic ability of HDL to be oxidized by measuring increasing fluorescence due to DHR oxidation over time. The assay distinguishes the oxidative potential of HDL taken from different persons, and the results are reproducible. Direct comparison of this measurement correlated well with results obtained using a validated cell-based assay (r(2) = 0.62, P < 0.001). The assay can be scaled from a 96-well format to a 384-well format and, therefore, is suitable for high-throughput implementation. This new fluorometric method offers an inexpensive, accurate, and rapid means for determining the oxidative properties of HDL that is applicable to large-scale clinical studies.
Figures


References
-
- Navab M., Berliner J. A., Watson A. D., Hama S. Y., Territo M. C., Lusis A. J., Shih D. M., Van Lenten B. J., Frank J. S., Demer L. L., et al. 1996. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler. Thromb. Vasc. Biol. 16: 831–842. - PubMed
-
- Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., et al. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41: 1481–1494. - PubMed
-
- Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., Fogelman A. M. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41: 1495–1508. - PubMed
-
- Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

