Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior
- PMID: 21957248
- PMCID: PMC3197228
- DOI: 10.1523/JNEUROSCI.1340-11.2011
Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior
Abstract
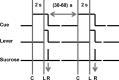
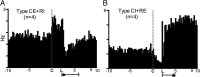
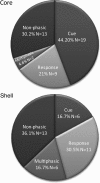
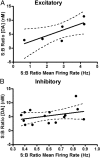
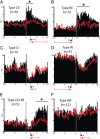
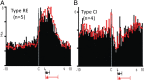
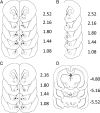
The mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) is critical in mediating reward-related behaviors, but the precise role of dopamine in this process remains unknown. We completed a series of studies to examine whether coincident changes occur in NAc cell firing and rapid dopamine release during goal-directed behaviors for sucrose and if so, to determine whether the two are causally linked. We show that distinct populations of NAc neurons differentially encode sucrose-directed behaviors, and using a combined electrophysiology/electrochemistry technique, further show that it is at those locations that rapid dopamine signaling is observed. To determine causality, NAc cell firing was recorded during selective pharmacological inactivation of dopamine burst firing using the NMDA receptor antagonist, AP-5. We show that phasic dopamine selectively modulates excitatory but not inhibitory responses of NAc neurons during sucrose-seeking behavior. Thus, rapid dopamine signaling does not exert global actions in the NAc but selectively modulates discrete NAc microcircuits that ultimately influence goal-directed actions.
Figures


References
-
- Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;35:238–242. - PubMed
-
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs ‘natural’ reinforcement. Physiol Behav. 2002;76:379–387. - PubMed
-
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
