The cortical rhythms of chronic back pain
- PMID: 21957259
- PMCID: PMC3214084
- DOI: 10.1523/JNEUROSCI.1984-11.2011
The cortical rhythms of chronic back pain
Abstract
Chronic pain is maladaptive and influences brain function and behavior by altering the flow and integration of information across brain regions. Here we use a power spectral analysis to investigate impact of presence of chronic pain on brain oscillatory activity in humans. We examine changes in BOLD fluctuations, across different frequencies, in chronic back pain (CBP) patients (n = 15) as compared to healthy controls (n = 15) during resting-state fMRI. While healthy subjects exhibited a specific, frequency band-dependent, large-scale neural organization, patients showed increased high-frequency BOLD oscillations (0.12-0.20 Hz) circumscribed mainly to medial prefrontal cortex (mPFC) and parts of the default mode network. In the patients a correlation analysis related the mPFC aberrant BOLD high-frequency dynamics to altered functional connectivity to pain signaling/modulating brain regions, thus linking BOLD frequency changes to function. We also found that increased frequency fluctuations within the mPFC were temporally synchronous with spontaneous pain changes in patients during a pain-rating task. These observations provide novel insights about the nature of CBP, identifying how it disturbs the resting brain, and link high-frequency BOLD oscillations to perception.
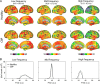
Figures



References
-
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004b;108:129–136. - PubMed
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
