Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL
- PMID: 21957301
- PMCID: PMC3233123
- DOI: 10.1128/JVI.05580-11
Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL
Abstract
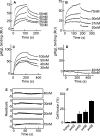
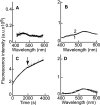
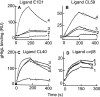
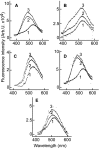
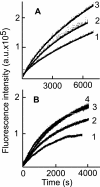
Fusion of herpesviruses with their target cells requires a minimum of three glycoproteins, namely, gB and a complex of gH and gL. Epstein-Barr virus (EBV) fusion with an epithelial cell requires no additional virus glycoproteins, and we have shown previously that it can be initiated by an interaction between integrin αvβ6 or αvβ8 and gHgL. We now report that integrin αvβ5 can also bind to gHgL and trigger fusion. Binding of gHgL to integrins is a two-step reaction. The first step, analyzed by surface plasmon resonance, was fast, with high association and low dissociation rate constants. The second step, detected by fluorescence spectroscopy of gHgL labeled at cysteine 153 at the domain I-domain II interface with the environmentally sensitive probes acrylodan and IANBD, involved a slower conformational change. Interaction of gHgL with neutralizing monoclonal antibodies or Fab' fragments was also consistent with a two-step reaction involving fast high-affinity binding and a subsequent slower conformational change. None of the antibodies bound to the same epitope, and none completely inhibited integrin binding. However, binding of each decreased the rate of conformational change induced by integrin binding, suggesting that neutralization might involve a conformational change that precludes fusion. Overall, the data are consistent with the interaction of gHgL with an integrin inducing a functionally important rearrangement at the domain I-domain II interface.
Figures




References
-
- Borza C. M., Hutt-Fletcher L. M. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594–599 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

