Molecular determinants of arenavirus Z protein homo-oligomerization and L polymerase binding
- PMID: 21957305
- PMCID: PMC3209362
- DOI: 10.1128/JVI.05691-11
Molecular determinants of arenavirus Z protein homo-oligomerization and L polymerase binding
Abstract
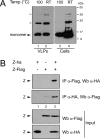
The arenavirus Z is a zinc-binding RING protein that has been implicated in multiple functions during the viral life cycle. These roles of Z involve interactions with viral and cellular proteins that remain incompletely understood. In this regard, Z inhibits viral RNA transcription and replication through direct interaction with the viral L polymerase. Here, we defined the L-binding domain of Tacaribe virus (TCRV) Z protein and the structural requirements mediating Z homo-oligomerization. By using site-directed mutagenesis, coimmunoprecipitation, and functional assays, we showed that residues R37, N39, W44, L50, and Y57, located around the zinc coordination site I, play a critical role in the Z-L interaction. We also found that Z protein from either TCRV or the pathogenic Junin virus (JUNV) self-associates into oligomeric forms in mammalian cells. Importantly, mutation of the myristoylation site, the strictly conserved residue G at position 2, severely impaired the ability of both TCRV Z and JUNV Z to self-interact as well as their capacity to accumulate at the plasma membrane, strongly suggesting that Z homo-oligomerization is associated with its myristoylation and cell membrane targeting. In contrast, disruption of the RING structure or substitution of W44 or N39, which are critical for L protein recognition, did not affect Z self-binding. Overall, the data presented here indicate that homo-oligomerization is not a requirement for Z-L interaction or Z-mediated polymerase activity inhibition.
Figures




References
-
- Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with Image J. Biophotonics Int. 11:36–42
-
- Buchmeier M. J., de La Torre J. C., Peters C. J. 2007. Arenaviridae: the viruses and their replication, p. 1791–1828 In Knipe D. M., et al. (ed.), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

