High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis
- PMID: 21957881
- PMCID: PMC3752281
- DOI: 10.1111/j.1365-2036.2011.04863.x
High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis
Abstract
Background: Ursodeoxycholic acid (UDCA) in a dose of 28-30 mg/kg/day increases the likelihood of clinical deterioration of primary sclerosing cholangitis (PSC) patients.
Aim: To compare the risk of adverse clinical endpoints in patients with varying disease status.
Methods: We reviewed records from patients previously enrolled in a study evaluating the effects of high dose (28-30 mg/kg/day) UDCA in PSC. Patients were grouped according to treatment (UDCA vs. placebo) and baseline disease status (histological stage of PSC, total serum bilirubin). Development of clinical endpoints including death, liver transplantation, cirrhosis, oesophageal varices and cholangiocarcinoma was sought.
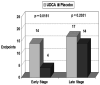
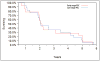
Results: A total of 150 patients were included of whom 49 patients developed endpoints. There was an increased development of endpoints among patients using UDCA vs. placebo (14 vs. 4, P=0.0151) with early histological disease (stage 1-2, n=88) but not with late stage (stage 3-4, n=62) disease (17 vs. 14, P=0.2031). Occurrence of clinical endpoints was also higher in patients receiving UDCA vs. placebo (16 vs. 2, P=0.0008) with normal bilirubin levels (total bilirubin ≤1.0 mg/dL) but not in patients with elevated bilirubin levels (15 vs. 16, P=0.6018). Among patients not reaching endpoints 31.7% had normalisation of their alkaline phosphatase levels when compared to 14.3% in patients who reached endpoints (P=0.073).
Conclusion: The increased risk of adverse events with UDCA treatment when compared with placebo is only apparent in patients with early histological stage disease or normal total bilirubin.
© 2011 Blackwell Publishing Ltd.
Figures

Comment in
-
Ursodeoxycholic acid in primary sclerosing cholangitis.Aliment Pharmacol Ther. 2012 Mar;35(5):622-3. doi: 10.1111/j.1365-2036.2011.04988.x. Aliment Pharmacol Ther. 2012. PMID: 22300127 No abstract available.
References
-
- Mendes F, Lindor KD. Primary sclerosing cholangitis: overview and update. Nat Rev Gastroenterol Hepatol. 2010;7(11):611–9. - PubMed
-
- Karlsen TH, Schrumpf E, Boberg KM. Primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2010;24(5):655–66. - PubMed
-
- Rudolph G, Gotthardt D, Kloeters-Plachky P, Rost D, Kulaksiz H, Stiehl A. In PSC with dominant bile duct stenosis, IBD is associated with an increase of carcinomas and reduced survival. J Hepatol. 2010;53(2):313–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
