Enzymatic methyl transfer: role of an active site residue in generating active site compaction that correlates with catalytic efficiency
- PMID: 21958159
- PMCID: PMC3219439
- DOI: 10.1021/ja207467d
Enzymatic methyl transfer: role of an active site residue in generating active site compaction that correlates with catalytic efficiency
Abstract
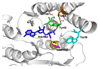
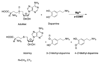
Human catechol-O-methyltransferase (COMT) catalyzes a methyl transfer from S-adenosylmethionine (AdoMet) to dopamine. Site-specific mutants at three positions (Tyr68, Trp38, and Val108) have been characterized with regard to product distribution, catalytic efficiency, and secondary kinetic isotope effects. The series of mutations at Tyr68 within wild-type protein and the common polymorphic variant (Val108Met) yields a linear correlation between the catalytic efficiency and the size of the secondary kinetic isotope effect. We conclude that active site compaction in COMT is modulated by a proximal side chain residing behind the sulfur-bearing methyl group of AdoMet. These findings are discussed in the context of the active site compression that has been postulated to accompany enzyme-supported hydrogen tunneling.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

