Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects
- PMID: 21958845
- PMCID: PMC3374485
- DOI: 10.1016/j.bone.2011.09.038
Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects
Abstract
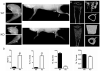
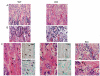
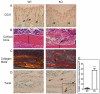
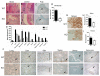
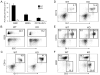
CSF-1, a key regulator of mononuclear phagocyte production, is highly expressed in the skeleton by osteoblasts/osteocytes and in a number of nonskeletal tissues such as uterus, kidney and brain. The spontaneous mutant op/op mouse has been the conventional model of CSF-1 deficiency and exhibits a pleiotropic phenotype characterized by osteopetrosis, and defects in hematopoiesis, fertility and neural function. Studies to further delineate the biologic effect of CSF-1 within various tissues have been hampered by the lack of suitable models. To address this issue, we generated CSF-1 floxed/floxed mice and demonstrate that Cre-mediated recombination using Meox2Cre, a Cre line expressed in epiblast during early embryogenesis, results in mice with ubiquitous CSF-1 deficiency (CSF-1KO). Homozygous CSF-1KO mice lacked CSF-1 in all tissues and displayed, in part, a similar phenotype to op/op mice that included: failure of tooth eruption, osteopetrosis, reduced macrophage densities in reproductive and other organs and altered hematopoiesis with decreased marrow cellularity, circulating monocytes and B cell lymphopoiesis. In contrast to op/op mice, CSF-1KO mice showed elevated circulating and splenic T cells. A striking feature in CSF-1KO mice was defective osteocyte maturation, bone mineralization and osteocyte-lacunar system that was associated with reduced dentin matrix protein 1 (DMP1) expression in osteocytes. CSF-1KO mice also showed a dramatic reduction in osteomacs along the endosteal surface that may have contributed to the hematopoietic and cortical bone defects. Thus, our findings show that ubiquitous CSF-1 gene deletion using a Cre-based system recapitulates the expected osteopetrotic phenotype. Moreover, results point to a novel link between CSF-1 and osteocyte survival/function that is essential for maintaining bone mass and strength during skeletal development.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Yeung Y-G, Stanley ER. Proteomics approaches to the analysis of early events in colony-stimulating factor-1 signal transduction. Mol Cell Proteomics. 2003;2:1143–1155. - PubMed
-
- Price LKH, Choi HU, Rosenberg L, Stanley ER. The predominant form of secreted colony stimulating factor is a proteoglycan. J Biol Chem. 1992;267:2190–2199. - PubMed
-
- Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. - PubMed
-
- Heino TJ, Kurata K, Higaki H, Vaananen HK. Evidence of a role of osteocytes in the initiation of targeted remodeling. Technology and Health Care. 2009;17:49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

