Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial
- PMID: 21959045
- PMCID: PMC4012268
- DOI: 10.5732/cjc.011.10188
Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial
Abstract
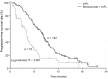
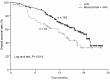
The efficacy and safety of bevacizumab with modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion (mIFL) in the first-line treatment of metastatic colorectal cancer (mCRC) has not been well evaluated in randomized clinical trials in Chinese patients. We conducted a phrase III trial in which patients with previously untreated mCRC were randomized 2:1 to the mIFL [irinotecan (125 mg/m(2)), leucovorin (20 mg/m(2)) bolus, and 5-fluorouracil intravenous infusion (500 mg/m(2)) weekly for four weeks every six weeks] plus bevacizumab (5 mg/kg every two weeks) group and the mIFL group, respectively. Co-primary objectives were progression-free survival (PFS) and 6-month PFS rate. In total, 214 patients were enrolled. Our results showed that addition of bevacizumab to mIFL significantly improved median PFS (4.2 months in the mIFL group vs. 8.3 months in the bevacizumab plus mIFL group, P < 0.001), 6-month PFS rate (25.0% vs. 62.6%, P < 0.001), median overall survival (13.4 months vs. 18.7 months, P = 0.014), and response rate (17% vs. 35%, P = 0.013). Grades 3 and 4 adverse events included diarrhea (21% in the mIFL group and 26% in the bevacizumab plus mIFL group) and neutropenia (19% in the mIFL group and 33% in the bevacizumab plus mIFL group). No wound-healing complications or congestive heart failure occurred. Our results suggested that bevacizumab plus mIFL is effective and well tolerated as first-line treatment for Chinese patients with mCRC. Clinical benefit and safety profiles were consistent with those observed in pivotal phase III trials with mainly Caucasian patients.
Figures
References
-
- Globocan 2008. Cancer incidence and mortality worldwide in 2008. Available at: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=160#BOTH. Accessed on 5th August, 2010.
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer [J] N Engl J Med. 2004;350(23):2335–2342. - PubMed
-
- Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer [J] J Clin Oncol. 2005;23(16):3706–3712. - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study [J] J Clin Oncol. 2008;26(12):2013–2019. Errata in: J Clin Oncol, 2008,26(18):3110; J Clin Oncol, 2009, 27(4):653. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200 [J] J Clin Oncol. 2007;25(12):1539–1544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
