Coordinating growth and maturation - insights from Drosophila
- PMID: 21959165
- PMCID: PMC4353487
- DOI: 10.1016/j.cub.2011.06.033
Coordinating growth and maturation - insights from Drosophila
Abstract
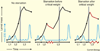
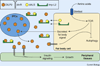
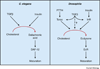
Adult body size in higher animals is dependent on the amount of growth that occurs during the juvenile stage. The duration of juvenile development, therefore, must be flexible and responsive to environmental conditions. When immature animals experience environmental stresses such as malnutrition or disease, maturation can be delayed until conditions improve and normal growth can resume. In contrast, when animals are raised under ideal conditions that promote rapid growth, internal checkpoints ensure that maturation does not occur until juvenile development is complete. Although the mechanisms that regulate growth and gate the onset of maturation have been investigated for decades, the emerging links between childhood obesity, early onset puberty, and adult metabolic disease have placed a new emphasis on this field. Remarkably, genetic studies in the fruit fly Drosophila melanogaster have shown that the central regulatory pathways that control growth and the timing of sexual maturation are conserved through evolution, and suggest that this aspect of animal life history is regulated by a common genetic architecture. This review focuses on these conserved mechanisms and highlights recent studies that explore how Drosophila coordinates developmental growth with environmental conditions.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Robertson CW. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J. Morphol. 1936;59:351–399.
-
- Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev. Cell. 2001;1:453–465. - PubMed
-
- Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam. Horm. 2001;60:1–73. - PubMed
-
- Gilbert LI, Warren JT. A molecular genetic approach to the biosynthesis of the insect steroid molting hormone. Vitam. Horm. 2005;73:31–57. - PubMed
-
- Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. J. Genet. Genomics. 2008;35:1–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

