The effect of polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair) on oral microbial colonization and pain control compared with other rinsing solutions in patients with oral mucositis after allogeneic stem cells transplantation
- PMID: 21959611
- PMCID: PMC3539478
- DOI: 10.12659/msm.881983
The effect of polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair) on oral microbial colonization and pain control compared with other rinsing solutions in patients with oral mucositis after allogeneic stem cells transplantation
Abstract
Background: Gelclair is an oral lubricating gel used in the management of oral mucositis (OM). We evaluated its efficacy, tolerance and impact on oral cavity microbial colonization in patients with OM after allogeneic hematopoietic stem cells transplantation.
Material/method: Gelclair was administered in a group of 22 patients with active OM. A control group of 15 patients used other rinsing solutions (chlorhexidine, benzydamine, salvia). Tests with oral cavity swabs for microbiology analysis were performed once a week.
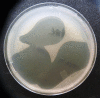
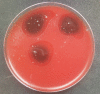
Results: The characteristics of OM in both groups were comparable, and rinsing solutions had satisfactory tolerability. There was no difference in the median improvement of oral intake and OM-related pain relief, which was assessed mostly as "slight effect". In the Gelclair group, the effect duration was longer (median 3 [0-5] vs. 1 [0-3] hours, p = 0.001). There was significant increase of Enterococcus faecalis and Candida sp. colonization of the oral cavity over the course of the hospitalization and significantly reduced incidence of such colonization in patients with OM in the Gelclair group: 1/22 (5%) vs. 6/15 (40%), p = 0.01. In vitro tests showed inhibited growth of Enterococcus faecalis and Candida sp. colonies within the area of the Gelclair application.
Conclusions: Gelclair may be individually helpful in the management of OM and pain in patients after allogeneic stem cells transplantation. Its use did not lead to worsened oral bacterial and yeast colonization and probably even helped to protect mucosa from Enterococcus and Candida sp. Further studies based on larger cohorts are needed.
Figures
References
-
- Barber C, Powell R, Ellis A, et al. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: a preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support Care Cancer. 2007;15:427–40. - PubMed
-
- Bonassi L, Cotroneo G, Nastasi G, et al. Treatment with Gelclair in patients suffering grade III–IV oral mucositis: efficacy and impact on quality of life (QOL) Anna Oncol. 2003;14:58.
-
- Flook C, Calman F, Mant M, et al. Gelclair vs benzydamine in randomise controlled study in patients with oral mucositis due to radical radiotherapy. Support Care Cancer. 2005;13:443–444.
-
- Innocenti M, Moscatelli G, Lopez S. Efficacy of Gelclair in reducing pain in palliative care patients with oral lesions: preliminary finding from an open pilot study. J Pain Symptom Manag. 2002;24:456–57. - PubMed
-
- Buchsel PC. Polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair): a bioadherent oral gel for the treatment of oral mucositis and other painful oral lesions. Expert Opin Drug Metab Toxicol. 2008;4:1449–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources