Molecular epidemical characteristics of Lamivudine resistance mutations of HBV in southern China
- PMID: 21959623
- PMCID: PMC3539467
- DOI: 10.12659/msm.881965
Molecular epidemical characteristics of Lamivudine resistance mutations of HBV in southern China
Abstract
Background: Lamivudine (LMV), as the preferred oral drug for use in treatment of HBV, always results in development of resistance mutations after long-term treatment. In this study we investigated chronic hepatitis B (CHB) patients in southern China to determine whether different HBV genotypes affect the incidence of LMV resistance mutations.
Material/methods: The study recruited 185 CHB patients living in southern China. Enzyme-linked immunosorbent assay was used to test for HBV serological markers, and HBV DNA was quantified by real-time PCR. Sequencing was performed to detect HBV genotypes and mutations.
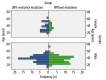
Results: There were 49.19% (91/185) CHB patients with HBV resistant to LMV. Only 2 genotypes were found: B and C; 62.16% (115/185) of patients were infected with genotype B HBV and 37.84% (70/185) of patients were infected with genotype C HBV. The incidence rate of LMV resistance was not significantly different between genotype B and C (49.57% vs. 48.57%, P>0.05). For the mean age and sex ratio, no significant difference was found. The pattern of rtM204I alone was predominantly observed (36.26%, 33/91), followed by rtM204V+rtL180M (23.08%, 21/91). The overall incidence rate of rtM204I mutation in genotype B (45.61%, 26/57) was more frequent than that in genotype C (20.59%, 7/34) (45.61% vs. 20.59%, P<0.05), but the incidence rate of other mutation patterns was not significantly different between genotypes B and C.
Conclusions: Our results emphasize that a LMV resistance test before treatment is of great importance in rational and optimal CHB therapy.
Figures
References
-
- WHO. Hepatitis B. [Accessed 16/9/2010]. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/
-
- WHO. Introduction of Hepatitis B Vaccine into Childhood Immunization Services. Geneva: WHO; 2001.
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. - PubMed
-
- Colacino JM, Staschke KA. The identification and development of antiviral agents for the treatment of chronic hepatitis B virus infection. Prog Drug Res. 1998;50:259–322. - PubMed
-
- Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, Salmeron J. Increased risk of autoimmune thyroid disease in hepatitis C vs. hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158:1445–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical