Randomised, phase III trial of epoetin-β to treat chemotherapy-induced anaemia according to the EU regulation
- PMID: 21959870
- PMCID: PMC3241560
- DOI: 10.1038/bjc.2011.395
Randomised, phase III trial of epoetin-β to treat chemotherapy-induced anaemia according to the EU regulation
Abstract
Background: Erythropoietin-stimulating agents (ESAs) effectively decrease the transfusion requirements of patients with chemotherapy-induced anaemia (CIA). Recent studies indicate that ESAs increase mortality and accelerate tumour progression. The studies also identify a 1.6-fold increased risk of venous thromboembolism. The ESA labelling was thus revised in Europe and the United States in 2008. This is the first randomised, phase III trial evaluating the efficacy and safety of epoetin-β (EPO), an ESA, dosed in accordance with the revised labelling, which specifies that ESAs should be administered to CIA patients with a haemoglobin level of ≤ 10 g dl⁻¹ and that a sustained haemoglobin level of > 12 g dl⁻¹ should be avoided.
Methods: A total of 186 CIA patients (8.0 g dl⁻¹ ≤ haemoglobin ≤ 10.0 g dl⁻¹) with lung or gynaecological cancer were randomised to receive EPO 36,000 IU or placebo weekly for 12 weeks.
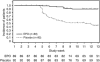
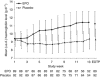
Results: The proportion of patients receiving transfusions or with haemoglobin < 8.0 g dl⁻¹ between week 5 and the end of the treatment period as the primary end point was significantly lower in the EPO group (n=89) than in the placebo group (n=92; 10.0% vs 56.4%, P < 0.001). The proportion receiving transfusions was significantly lower in the EPO group (4.5% vs 19.6%, P=0.002). Changes in quality of life were not different. No significant differences in adverse events - for example, the incidence of thromboembolic events was 1.1% for each group - or the 1-year overall survival were observed between groups.
Conclusion: Weekly EPO administered according to the revised labelling approved by the European Medicines Agency is effective and well tolerated for CIA treatment. Further investigations are needed on the effect of ESAs on mortality.
Figures
References
-
- Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, Mayordomo J, Reichert D, Pedrini JL, Ukarma L, Scherhag A, Burger HU (2008a) Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Cin Oncol 26: 592–598 - PubMed
-
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kuzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M (2008) Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 299: 914–924 - PubMed
-
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A (2009a) Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomized trials. Lancet 373: 1532–1542 - PubMed
-
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A (2009b) Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomized trials. Erratum in Lancet 374: 28 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials