Fas death receptor signalling: roles of Bid and XIAP
- PMID: 21959933
- PMCID: PMC3252833
- DOI: 10.1038/cdd.2011.121
Fas death receptor signalling: roles of Bid and XIAP
Abstract
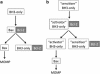
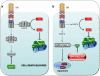
Fas (also called CD95 or APO-1), a member of a subgroup of the tumour necrosis factor receptor superfamily that contain an intracellular death domain, can initiate apoptosis signalling and has a critical role in the regulation of the immune system. Fas-induced apoptosis requires recruitment and activation of the initiator caspase, caspase-8 (in humans also caspase-10), within the death-inducing signalling complex. In so-called type 1 cells, proteolytic activation of effector caspases (-3 and -7) by caspase-8 suffices for efficient apoptosis induction. In so-called type 2 cells, however, killing requires amplification of the caspase cascade. This can be achieved through caspase-8-mediated proteolytic activation of the pro-apoptotic Bcl-2 homology domain (BH)3-only protein BH3-interacting domain death agonist (Bid), which then causes mitochondrial outer membrane permeabilisation. This in turn leads to mitochondrial release of apoptogenic proteins, such as cytochrome c and, pertinent for Fas death receptor (DR)-induced apoptosis, Smac/DIABLO (second mitochondria-derived activator of caspase/direct IAP binding protein with low Pi), an antagonist of X-linked inhibitor of apoptosis (XIAP), which imposes a brake on effector caspases. In this review, written in honour of Juerg Tschopp who contributed so much to research on cell death and immunology, we discuss the functions of Bid and XIAP in the control of Fas DR-induced apoptosis signalling, and we speculate on how this knowledge could be exploited to develop novel regimes for treatment of cancer.
Figures

References
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
-
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. - PubMed
-
- Vanden Berghe T, van Loo G, Saelens X, Van Gurp M, Brouckaert G, Kalai M, et al. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. J Biol Chem. 2004;279:7925–7933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

