Effect of synthetic cationic protein on mechanoexcitability of vagal afferent nerve subtypes in guinea pig esophagus
- PMID: 21960520
- PMCID: PMC3233783
- DOI: 10.1152/ajpgi.00015.2011
Effect of synthetic cationic protein on mechanoexcitability of vagal afferent nerve subtypes in guinea pig esophagus
Abstract
Eosinophilic esophagitis is characterized by increased infiltration and degranulation of eosinophils in the esophagus. Whether eosinophil-derived cationic proteins regulate esophageal sensory nerve function is still unknown. Using synthetic cationic protein to investigate such effect, we performed extracellular recordings from vagal nodose or jugular neurons in ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus. Nerve excitabilities were determined by comparing action potentials evoked by esophageal distensions before and after perfusion of synthetic cationic protein poly-L-lysine (PLL) with or without pretreatment with poly-L-glutamic acid (PLGA), which neutralized cationic charges of PLL. Perfusion with PLL did not evoke action potentials in esophageal nodose C fibers but increased their responses to esophageal distension. This potentiation effect lasted for 30 min after washing out of PLL. Pretreatment with PLGA significantly inhibited PLL-induced mechanohyperexcitability of esophageal nodose C fibers. In esophageal nodose Aδ fibers, perfusion with PLL did not evoke action potentials. In contrast to nodose C fibers, both the spontaneous discharges and the responses to esophageal distension in nodose Aδ fibers were decreased by perfusion with PLL, which can be restored after washing out PLL for 30-60 min. Pretreatment with PLGA attenuated PLL-induced decrease in spontaneous discharge and mechanoexcitability of esophageal nodose Aδ fibers. In esophageal jugular C fibers, PLL neither evoked action potentials nor changed their responses to esophageal distension. Collectively, these data demonstrated that synthetic cationic protein did not evoke action potential discharges of esophageal vagal afferents but had distinctive sensitization effects on their responses to esophageal distension.
Figures


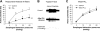

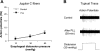
References
-
- Achem SR, Almansa C, Krishna M, Heckman MG, Wolfsen HC, Talley NJ, DeVault KR. Oesophageal eosinophilic infiltration in patients with noncardiac chest pain. Aliment Pharmacol Ther 33: 1194–1201, 2011 - PubMed
-
- Allende DS, Yerian LM. Diagnosing gastroesophageal reflux disease: the pathologist's perspective. Adv Anat Pathol 16: 161–165, 2009Review - PubMed
-
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19, Suppl 1: 1–19, 2007 - PubMed
-
- Coyle AJ, Ackerman SJ, Irvin CG. Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am Rev Respir Dis 147: 896–900, 1993 - PubMed
-
- Durcan N, Costello RW, McLean WG, Blusztajn J, Madziar B, Fenech AG, Hall IP, Gleich GJ, McGarvey L, Walsh MT. Eosinophil-mediated cholinergic nerve remodeling. Am J Respir Cell Mol Biol 34: 775–786, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

