Human retinal pigment epithelium cells as functional models for the RPE in vivo
- PMID: 21960553
- PMCID: PMC3208409
- DOI: 10.1167/iovs.11-8021
Human retinal pigment epithelium cells as functional models for the RPE in vivo
Abstract
Purpose: The two most commonly used in vitro models of the retinal pigment epithelium (RPE) are fetal human RPE (fhRPE) and ARPE-19 cells; however, studies of their barrier properties have produced contradictory results. To compare their utility as RPE models, their morphologic and functional characteristics were analyzed.
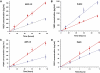
Methods: Monolayers of both cell types were grown on permeable membrane filters. Barrier function and cellular morphology were assessed by transepithelial resistance (TER) measurements and immunohistochemistry. Protein expression was evaluated by immunoblotting and ELISA assays, and retinoid metabolism characterized by HPLC.
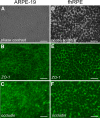
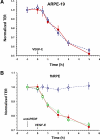
Results: Both cultures developed tight junctions. However, only the fhRPE cells were pigmented, uniform in size and shape, expressed high levels of RPE markers, metabolized all-trans retinal, and developed high TER (>400 Ωcm(2)). The net secretion of pigment-epithelium-derived factor (PEDF) was directed apically in both cultures, but fhRPE cells exhibited secretion rates a thousand-fold greater than in ARPE-19 cells. The net secretion of vascular endothelial growth factor (VEGF) was significantly higher in fhRPE cultures and the direction of this secretion was basolateral; while net secretion was apical in ARPE-19 cells. In fresh media, VEGF-E reduced TER in both cultures; however, in conditioned media fhRPE cells did not respond to VEGF-E administration, but retreatment of the conditioned media with anti-PEDF antibodies allowed fhRPE cells to fully respond to VEGF-E.
Conclusions: Properties of fhRPE cells align with a functionally normal RPE in vivo, while ARPE-19 cells resemble a pathologic or aged RPE. These results suggest a utility for both cell types in understanding distinct, particular aspects of RPE function.
Figures


Comment in
-
VEGF and PEDF secretion in ARPE-19 and fhRPE cells.Invest Ophthalmol Vis Sci. 2011 Nov 21;52(12):9047. doi: 10.1167/iovs.11-8737. Invest Ophthalmol Vis Sci. 2011. PMID: 22104197 Free PMC article. No abstract available.
References
-
- Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97:217–228 - PubMed
-
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901 - PubMed
-
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228 - PubMed
-
- Marmor MF. Mechanisms of fluid accumulation in retinal edema. Doc Ophthalmol. 1999;97:239–249 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY019065/EY/NEI NIH HHS/United States
- R01 EY019065/EY/NEI NIH HHS/United States
- AG022103/AG/NIA NIH HHS/United States
- R21 EY020661/EY/NEI NIH HHS/United States
- EY13160/EY/NEI NIH HHS/United States
- R01 AG022103/AG/NIA NIH HHS/United States
- R56 EY013160/EY/NEI NIH HHS/United States
- R01 EY009741/EY/NEI NIH HHS/United States
- EY020661/EY/NEI NIH HHS/United States
- R01 EY021368/EY/NEI NIH HHS/United States
- R01 EY013160/EY/NEI NIH HHS/United States
- EY009741/EY/NEI NIH HHS/United States
- T32 GM008716/GM/NIGMS NIH HHS/United States
- 2T32 GM008716-11/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

