Microparticle enlargement and altered surface proteins after air decompression are associated with inflammatory vascular injuries
- PMID: 21960660
- PMCID: PMC3290415
- DOI: 10.1152/japplphysiol.00953.2011
Microparticle enlargement and altered surface proteins after air decompression are associated with inflammatory vascular injuries
Abstract
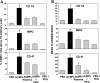
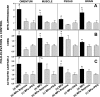
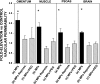
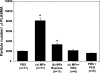
Studies in a murine model have shown that decompression stress triggers a progressive elevation in the number of circulating annexin V-coated microparticles derived from leukocytes, erythrocytes, platelets, and endothelial cells. We noted that some particles appeared to be larger than anticipated, and size continued to increase for ≥24 h postdecompression. These observations led to the hypothesis that inert gas bubbles caused the enlargement and particle size could be reduced by hydrostatic pressure. After demonstrating pressure-induced particle size reduction, we hypothesized that annexin V-positive particle changes associated with decompression contributed to their proinflammatory potential. Intravenous injection of naive mice with particles isolated from decompressed mice, but not control mice, caused intravascular neutrophil activation; perivascular neutrophil sequestration and tissue injuries were documented as elevations of vascular permeability and activated caspase-3. These changes were not observed if mice were injected with particles that had been subjected to hydrostatic recompression or particles that had been emulsified by incubation with polyethylene glycol telomere B surfactant. Hydrostatic pressure and surfactant incubation also altered the pattern of proteins expressed on the surface of particles. We conclude that proinflammatory events and vascular damage are due to enlargement of annexin V-coated particles and/or changes in surface marker protein pattern associated with provocative decompression. Injection of annexin V-coated particles from decompressed mice will recapitulate the pathophysiological vascular changes observed following decompression stress.
Figures



References
-
- Altman PL, Dittmer DS. Biology Databook. Washington, DC: FASEB, 1964, p. 264
-
- Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost 103: 1044–1052, 2010 - PubMed
-
- Eftedal OS, Lydersen S, Brubakk AO. The relationship between venous gas bubbles and adverse effects of decompression after air dives. Undersea Hyperb Med 34: 99–105, 2007 - PubMed
-
- Enjeti AK, Lincz LF, Seldon M. Detection and measurement of microparticles: an evolving research tool for vascular biology. Semin Thromb Hemost 33: 771–779, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

