A Mycobacterium leprae Hsp65 mutant as a candidate for mitigating lupus aggravation in mice
- PMID: 21961033
- PMCID: PMC3178518
- DOI: 10.1371/journal.pone.0024093
A Mycobacterium leprae Hsp65 mutant as a candidate for mitigating lupus aggravation in mice
Abstract
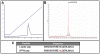
Hsp60 is an abundant and highly conserved family of intracellular molecules. Increased levels of this family of proteins have been observed in the extracellular compartment in chronic inflammation. Administration of M. leprae Hsp65 [WT] in [NZBxNZW]F(1) mice accelerates the Systemic Lupus Erythematosus [SLE] progression whereas the point mutated K(409)A Hsp65 protein delays the disease. Here, the biological effects of M. leprae Hsp65 Leader pep and K(409)A pep synthetic peptides, which cover residues 352-371, are presented. Peptides had immunomodulatory effects similar to that observed with their respective proteins on survival and the combined administration of K(409)A+Leader pep or K(409)A pep+WT showed that the mutant forms were able to inhibit the deleterious effect of WT on mortality, indicating the neutralizing potential of the mutant molecules in SLE progression. Molecular modeling showed that replacing Lysine by Alanine affects the electrostatic potential of the 352-371 region. The number of interactions observed for WT is much higher than for Hsp65 K(409)A and mouse Hsp60. The immunomodulatory effects of the point-mutated protein and peptide occurred regardless of the catalytic activity. These findings may be related to the lack of effect on survival when F(1) mice were inoculated with Hsp60 or K(409)A pep. Our findings indicate the use of point-mutated Hsp65 molecules, such as the K(409)A protein and its corresponding peptide, that may minimize or delay the onset of SLE, representing a new approach to the treatment of autoimmune diseases.
Conflict of interest statement
Figures



References
-
- Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. - PubMed
-
- Georgopoulos C. The emergence of the chaperone machines. Trends Biochem Sci. 1992;17:295–299. - PubMed
-
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. - PubMed
-
- Perschinka H, Mayr M, Millonig G, Mayerl C, van der Zee R, et al. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1060–1065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

