Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala
- PMID: 21961035
- PMCID: PMC3178530
- DOI: 10.1371/journal.pone.0024349
Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala
Abstract
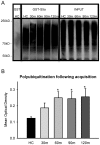
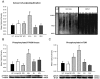
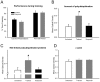
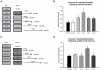
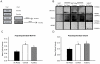
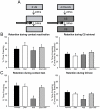
Protein degradation through the ubiquitin-proteasome system [UPS] plays a critical role in some forms of synaptic plasticity. However, its role in memory formation in the amygdala, a site critical for the formation of fear memories, currently remains unknown. Here we provide the first evidence that protein degradation through the UPS is critically engaged at amygdala synapses during memory formation and retrieval. Fear conditioning results in NMDA-dependent increases in degradation-specific polyubiquitination in the amygdala, targeting proteins involved in translational control and synaptic structure and blocking the degradation of these proteins significantly impairs long-term memory. Furthermore, retrieval of fear memory results in a second wave of NMDA-dependent polyubiquitination that targets proteins involved in translational silencing and synaptic structure and is critical for memory updating following recall. These results indicate that UPS-mediated protein degradation is a major regulator of synaptic plasticity necessary for the formation and stability of long-term memories at amygdala synapses.
Conflict of interest statement
Figures
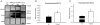
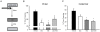
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

