Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells
- PMID: 21961046
- PMCID: PMC3178553
- DOI: 10.1371/journal.pone.0024807
Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells
Abstract
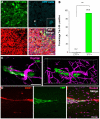
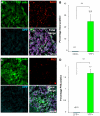
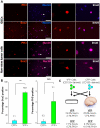
High-grade gliomas (World Health Organization grade III anaplastic astrocytoma and grade IV glioblastoma multiforme), the most prevalent primary malignant brain tumors, display a cellular hierarchy with self-renewing, tumorigenic cancer stem cells (CSCs) at the apex. While the CSC hypothesis has been an attractive model to describe many aspects of tumor behavior, it remains controversial due to unresolved issues including the use of ex vivo analyses with differential growth conditions. A CSC population has been confirmed in malignant gliomas by preferential tumor formation from cells directly isolated from patient biopsy specimens. However, direct comparison of multiple tumor cell populations with analysis of the resulting phenotypes of each population within a representative tumor environment has not been clearly described. To directly test the relative tumorigenic potential of CSCs and non-stem tumor cells in the same microenvironment, we interrogated matched tumor populations purified from a primary human tumor transplanted into a xenograft mouse model and monitored competitive in vivo tumor growth studies using serial in vivo intravital microscopy. While CSCs were a small minority of the initial transplanted cancer cell population, the CSCs, not the non-stem tumor cells, drove tumor formation and yielded tumors displaying a cellular hierarchy. In the resulting tumors, a fraction of the initial transplanted CSCs maintained expression of stem cell and proliferation markers, which were significantly higher compared to the non-stem tumor cell population and demonstrated that CSCs generated cellular heterogeneity within the tumor. These head-to-head comparisons between matched CSCs and non-stem tumor cells provide the first functional evidence using live imaging that in the same microenvironment, CSCs more than non-stem tumor cells are responsible for tumor propagation, confirming the functional definition of a CSC.
Conflict of interest statement
Figures


References
-
- Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–361. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

