Chain collapse of an amyloidogenic intrinsically disordered protein
- PMID: 21961598
- PMCID: PMC3183800
- DOI: 10.1016/j.bpj.2011.08.024
Chain collapse of an amyloidogenic intrinsically disordered protein
Abstract
Natively unfolded or intrinsically disordered proteins (IDPs) are under intense scrutiny due to their involvement in both normal biological functions and abnormal protein misfolding disorders. Polypeptide chain collapse of amyloidogenic IDPs is believed to play a key role in protein misfolding, oligomerization, and aggregation leading to amyloid fibril formation, which is implicated in a number of human diseases. In this work, we used bovine κ-casein, which serves as an archetypal model protein for amyloidogenic IDPs. Using a variety of biophysical tools involving both prediction and spectroscopic techniques, we first established that monomeric κ-casein adopts a collapsed premolten-globule-like conformational ensemble under physiological conditions. Our time-resolved fluorescence and light-scattering data indicate a change in the mean hydrodynamic radius from ∼4.6 nm to ∼1.9 nm upon chain collapse. We then took the advantage of two cysteines separated by 77 amino-acid residues and covalently labeled them using thiol-reactive pyrene maleimide. This dual-labeled protein demonstrated a strong excimer formation upon renaturation from urea- and acid-denatured states under both equilibrium and kinetic conditions, providing compelling evidence of polypeptide chain collapse under physiological conditions. The implication of the IDP chain collapse in protein aggregation and amyloid formation is also discussed.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

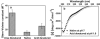




References
-
- Dunker A.K., Lawson J.D., Obradovic Z. Intrinsically disordered protein. J. Mol. Graph. Model. 2001;19:26–59. - PubMed
-
- Tompa P. CRC Press; Boca Raton, FL: 2010. Structure and Function of Intrinsically Disordered Proteins.
-
- Uversky V.N. Intrinsically disordered proteins and their environment: effects of strong denaturants, temperature, pH, counter ions, membranes, binding partners, osmolytes, and macromolecular crowding. Protein J. 2009;28:305–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

