Kinetic, mechanistic, and structural modeling studies of truncated wild-type leucine-rich repeat kinase 2 and the G2019S mutant
- PMID: 21961647
- PMCID: PMC3205922
- DOI: 10.1021/bi201173d
Kinetic, mechanistic, and structural modeling studies of truncated wild-type leucine-rich repeat kinase 2 and the G2019S mutant
Abstract
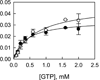
Leucine-rich repeat kinase 2 (LRRK2), a large and complex protein that possesses two enzymatic properties, kinase and GTPase, is one of the major genetic factors in Parkinson's disease (PD). Here, we characterize the kinetic and catalytic mechanisms of truncated wild-type (t-wt) LRRK2 and its most common mutant, G2019S (t-G2019S), with a structural interpretation of the kinase domain. First, the substitution of threonine with serine in the LRRKtide peptide results in a much less efficient substrate as demonstrated by a 26-fold decrease in k(cat) and a 6-fold decrease in binding affinity. The significant decrease in k(cat) is attributed to a slow chemical transfer step as evidenced by the inverse solvent kinetic isotope effect in the proton inventory and pL (pH or pD)-dependent studies. The shape of the proton inventory and pL profile clearly signals the involvement of a general base (pK(a) = 7.5) in the catalysis with a low fractionation factor in the ground state. We report for the first time that the increased kinase activity of the G2019S mutant is substrate-dependent. Homology modeling of the kinase domain (open and closed forms) and structural analysis of the docked peptide substrates suggest that electrostatic interactions play an important role in substrate recognition, which is affected by G2019S and may directly influence the kinetic properties of the enzyme. Finally, the GTPase activity of the t-G2019S mutant was characterized, and the mutation modestly decreases GTPase activity without significantly affecting GTP binding affinity.
Figures




References
-
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. - PubMed
-
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. - PubMed
-
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. - PubMed
-
- Berg D, Schweitzer KJ, Leitner P, Zimprich A, Lichtner P, Belcredi P, Brussel T, Schulte C, Maass S, Nagele T, Wszolek ZK, Gasser T. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease*. Brain. 2005;128:3000–3011. - PubMed
-
- Khan NL, Jain S, Lynch JM, Pavese N, Abou-Sleiman P, Holton JL, Healy DG, Gilks WP, Sweeney MG, Ganguly M, Gibbons V, Gandhi S, Vaughan J, Eunson LH, Katzenschlager R, Gayton J, Lennox G, Revesz T, Nicholl D, Bhatia KP, Quinn N, Brooks D, Lees AJ, Davis MB, Piccini P, Singleton AB, Wood NW. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

