SULT1A1, CYP2C19 and disease-free survival in early breast cancer patients receiving tamoxifen
- PMID: 21961651
- PMCID: PMC3235041
- DOI: 10.2217/pgs.11.97
SULT1A1, CYP2C19 and disease-free survival in early breast cancer patients receiving tamoxifen
Abstract
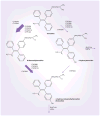
Aim: Tamoxifen biotransformation to endoxifen, a potent antiestrogen, is catalyzed by CYP2D6. In addition, CYP2C19 and SULT1A1 have also been implicated in the metabolism of tamoxifen. We sought to evaluate the importance of SULT1A1 copy number and CYP2C19*17 on disease-free survival (DFS) in postmenopausal women randomized to tamoxifen monotherapy in North Central Cancer Treatment Group 89-30-52 from January 1991 to April 1995.
Materials & methods: We extracted DNA from paraffin-embedded tumors and determined tumor SULT1A1 copy number and CYP2C19*17 genotype. The association of genotype with DFS was determined using the log-rank test. Multivariate cox modeling was performed using traditional prognostic factors, as well as CYP2D6 genotype. SULT1A1 copy number and CYP2C19*17 genotype was determined in 190 out of 256 patients (95% Caucasian).
Results: The median follow-up for living patients was 14 years. DFS did not differ according to SULT1A1 copy number (p = 0.482) or CYP2C19*17 genotype (p = 0.667). Neither SULT1A1 copy number or CYP2C19*17 genotype was associated with disease recurrence in this cohort.
Conclusion: Future studies are needed to identify whether other genetic and environmental factors which affect tamoxifen metabolism are associated with tamoxifen clinical outcomes.
Figures


References
-
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. - PubMed
-
- Lien EA, Anker G, Lonning PE, Solheim E, Ueland PM. Decreased serum concentrations of tamoxifen and its metabolites induced by aminoglutethimide. Cancer Res. 1990;50:5851–5857. - PubMed
-
- Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49:2175–2183. - PubMed
-
- Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991;51:4837–4844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
