A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes
- PMID: 21961694
- PMCID: PMC3203094
- DOI: 10.1186/1746-4811-7-30
A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes
Abstract
Background: Plant protoplasts, a proven physiological and versatile cell system, are widely used in high-throughput analysis and functional characterization of genes. Green protoplasts have been successfully used in investigations of plant signal transduction pathways related to hormones, metabolites and environmental challenges. In rice, protoplasts are commonly prepared from suspension cultured cells or etiolated seedlings, but only a few studies have explored the use of protoplasts from rice green tissue.
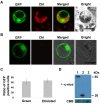
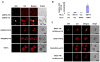
Results: Here, we report a simplified method for isolating protoplasts from normally cultivated young rice green tissue without the need for unnecessary chemicals and a vacuum device. Transfections of the generated protoplasts with plasmids of a wide range of sizes (4.5-13 kb) and co-transfections with multiple plasmids achieved impressively high efficiencies and allowed evaluations by 1) protein immunoblotting analysis, 2) subcellular localization assays, and 3) protein-protein interaction analysis by bimolecular fluorescence complementation (BiFC) and firefly luciferase complementation (FLC). Importantly, the rice green tissue protoplasts were photosynthetically active and sensitive to the retrograde plastid signaling inducer norflurazon (NF). Transient expression of the GFP-tagged light-related transcription factor OsGLK1 markedly upregulated transcript levels of the endogeneous photosynthetic genes OsLhcb1, OsLhcp, GADPH and RbcS, which were reduced to some extent by NF treatment in the rice green tissue protoplasts.
Conclusions: We show here a simplified and highly efficient transient gene expression system using photosynthetically active rice green tissue protoplasts and its broad applications in protein immunoblot, localization and protein-protein interaction assays. These rice green tissue protoplasts will be particularly useful in studies of light/chloroplast-related processes.
Figures






References
-
- De Sutter V, Vanderhaeghen R, Tilleman S, Lammertyn F, Vanhoutte I, Karimi M, Inzé D, Goossens A, Hilson P. Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J. 2005;44(6):1065–1076. doi: 10.1111/j.1365-313X.2005.02586.x. - DOI - PubMed
-
- Fischer R, Hain R. Tobacco protoplast transformation and use for functional analysis of newly isolated genes and gene constructs. Methods Cell Biol. 1995;50:401–410. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

