Two new "protected" oxyphors for biological oximetry: properties and application in tumor imaging
- PMID: 21961699
- PMCID: PMC3617485
- DOI: 10.1021/ac2022234
Two new "protected" oxyphors for biological oximetry: properties and application in tumor imaging
Abstract
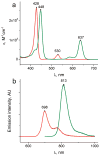
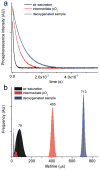
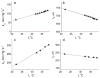
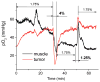
We report the synthesis, calibration, and examples of application of two new phosphorescent probes, Oxyphor R4 and Oxyphor G4, optimized specifically for in vivo oxygen imaging by phosphorescence quenching. These "protected" dendritic probes can operate in either albumin-rich (blood plasma) or albumin-free (interstitial space) environments at all physiological oxygen concentrations, from normoxic to deep hypoxic conditions. Oxyphors R4 and G4 are derived from phosphorescent Pd-meso-tetra-(3,5-dicarboxyphenyl)-porphyrin (PdP) or Pd-meso-tetra-(3,5-dicarboxyphenyl)-tetrabenzoporphyrin (PdTBP), respectively, and possess features common for protected dendritic probes, i.e., hydrophobic dendritic encapsulation of phosphorescent metalloporphyrins and hydrophilic PEGylated periphery. The new Oxyphors are highly soluble in aqueous environments and do not permeate biological membranes. The probes were calibrated under physiological conditions (pH 6.4-7.8) and temperatures (22-38 °C), showing high stability, reproducibility of signals, and lack of interactions with biological solutes. Oxyphor G4 was used to dynamically image intravascular and interstitial oxygenation in murine tumors in vivo. The physiological relevance of the measurements was demonstrated by dynamically recording changes in tissue oxygenation during application of anesthesia (isofluorane). These experiments revealed that changes in isofluorane concentration significantly affect tissue oxygenation.
Figures


Similar articles
-
Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence.Anal Biochem. 2002 Nov 15;310(2):191-8. doi: 10.1016/s0003-2697(02)00384-6. Anal Biochem. 2002. PMID: 12423638
-
Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence.J Appl Physiol (1985). 2005 Apr;98(4):1503-10. doi: 10.1152/japplphysiol.01140.2004. Epub 2004 Dec 3. J Appl Physiol (1985). 2005. PMID: 15579567
-
Oxygen pressures in the interstitial space of skeletal muscle and tumors in vivo.Adv Exp Med Biol. 2008;614:53-62. doi: 10.1007/978-0-387-74911-2_7. Adv Exp Med Biol. 2008. PMID: 18290314 Free PMC article.
-
Evaluation of phototoxicity of dendritic porphyrin-based phosphorescent oxygen probes: an in vitro study.Photochem Photobiol Sci. 2011 Jun;10(6):1056-65. doi: 10.1039/c0pp00356e. Epub 2011 Mar 15. Photochem Photobiol Sci. 2011. PMID: 21409208 Free PMC article.
-
Emerging applications of phosphorescent metalloporphyrins.J Fluoresc. 2005 Jul;15(4):569-84. doi: 10.1007/s10895-005-2830-x. J Fluoresc. 2005. PMID: 16167215 Review.
Cited by
-
Microfluidic device to attain high spatial and temporal control of oxygen.PLoS One. 2018 Dec 20;13(12):e0209574. doi: 10.1371/journal.pone.0209574. eCollection 2018. PLoS One. 2018. PMID: 30571786 Free PMC article.
-
Live-animal imaging of native haematopoietic stem and progenitor cells.Nature. 2020 Feb;578(7794):278-283. doi: 10.1038/s41586-020-1971-z. Epub 2020 Feb 5. Nature. 2020. PMID: 32025033 Free PMC article.
-
Long-wavelength analyte-sensitive luminescent probes and optical (bio)sensors.Methods Appl Fluoresc. 2015 Dec;3(4):042005. doi: 10.1088/2050-6120/3/4/042005. Epub 2015 Oct 22. Methods Appl Fluoresc. 2015. PMID: 27134748 Free PMC article.
-
Oxygen Consumption In Vivo by Ultra-High Dose Rate Electron Irradiation Depends Upon Baseline Tissue Oxygenation.Int J Radiat Oncol Biol Phys. 2025 Mar 15;121(4):1053-1062. doi: 10.1016/j.ijrobp.2024.10.018. Epub 2024 Oct 24. Int J Radiat Oncol Biol Phys. 2025. PMID: 39461597 Free PMC article.
-
Electrospun Fiber Mesh for High-Resolution Measurements of Oxygen Tension in Cranial Bone Defect Repair.ACS Appl Mater Interfaces. 2019 Sep 18;11(37):33548-33558. doi: 10.1021/acsami.9b08341. Epub 2019 Sep 4. ACS Appl Mater Interfaces. 2019. PMID: 31436082 Free PMC article.
References
-
- Yu DY, Cringle SJ. Exp Eye Res. 2005;80:745. - PubMed
-
- Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D. Int J Radiat Biol. 2006;82:699. - PubMed
-
- Blomgren K, Hagberg H. Free Radical Biol Med. 2006;40:388. - PubMed
-
- Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Br J Dermatol. 2010;163:257. - PubMed
-
- Brown JM. J Natl Cancer Inst. 1990;82:338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

