Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 and Ang-2 in rat brain
- PMID: 21962003
- PMCID: PMC3250229
- DOI: 10.1667/rr2647.1
Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 and Ang-2 in rat brain
Abstract
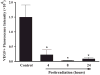
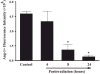
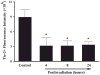
The etiology of radiation-induced cerebrovascular rarefaction remains unknown. In the present study, we examined the effect of whole-brain irradiation on endothelial cell (EC) proliferation/apoptosis and expression of various angiogenic factors in rat brain. F344 × BN rats received either whole-brain irradiation (a single dose of 10 Gy γ rays) or sham irradiation and were maintained for 4, 8 and 24 h after irradiation. Double immunofluorescence staining was employed to visualize EC proliferation/apoptosis in brain. The mRNA and protein expression levels of vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), endothelial-specific receptor tyrosine kinase (Tie-2), and Ang-2 in brain were determined by real-time RT-PCR and immunofluorescence staining. A significant reduction in CD31-immunoreactive cells was detected in irradiated rat brains compared with sham-irradiated controls. Whole-brain irradiation significantly suppressed EC proliferation and increased EC apoptosis. In addition, a significant decrease in mRNA and protein expression of VEGF, Ang-1 and Tie-2 was observed in irradiated rat brains. In contrast, whole-brain irradiation significantly upregulated Ang-2 expression in rat brains. The present study provides novel evidence that whole-brain irradiation differentially affects mRNA and protein expression of VEGF, Ang-1, Tie-2 and Ang-2. These changes are closely associated with decreased EC proliferation and increased EC apoptosis in brain.
Figures


Similar articles
-
Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis.Lab Invest. 2006 Nov;86(11):1172-84. doi: 10.1038/labinvest.3700476. Epub 2006 Sep 11. Lab Invest. 2006. PMID: 16969369
-
Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma.J Neurotrauma. 2005 May;22(5):518-28. doi: 10.1089/neu.2005.22.518. J Neurotrauma. 2005. PMID: 15892598
-
[Expression changes of Ang-1, Ang-2, Tie-2 and VEGF in co-culturing of endothelial cells and astrocytes after irradiation by X-ray and their significances].Zhonghua Yi Xue Za Zhi. 2014 Jun 17;94(23):1815-9. Zhonghua Yi Xue Za Zhi. 2014. PMID: 25154850 Chinese.
-
Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis.Am J Physiol Heart Circ Physiol. 2008 Jun;294(6):H2547-57. doi: 10.1152/ajpheart.01250.2007. Epub 2008 Apr 11. Am J Physiol Heart Circ Physiol. 2008. PMID: 18408125
-
Ang-1 and VEGF: central regulators of angiogenesis.Mol Cell Biochem. 2025 Feb;480(2):621-637. doi: 10.1007/s11010-024-05010-3. Epub 2024 Apr 23. Mol Cell Biochem. 2025. PMID: 38652215 Review.
Cited by
-
Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: evidence for microvascular injury in the cerebral white matter.Geroscience. 2023 Jun;45(3):1491-1510. doi: 10.1007/s11357-023-00735-3. Epub 2023 Feb 16. Geroscience. 2023. PMID: 36792820 Free PMC article.
-
Whole brain irradiation-induced endothelial dysfunction in the mouse brain.Geroscience. 2024 Feb;46(1):531-541. doi: 10.1007/s11357-023-00990-4. Epub 2023 Nov 13. Geroscience. 2024. PMID: 37953375 Free PMC article.
-
Radiation-induced brain injury: A review.Front Oncol. 2012 Jul 19;2:73. doi: 10.3389/fonc.2012.00073. eCollection 2012. Front Oncol. 2012. PMID: 22833841 Free PMC article.
-
An untapped window of opportunity for glioma: targeting therapy-induced senescence prior to recurrence.NPJ Precis Oncol. 2023 Nov 29;7(1):126. doi: 10.1038/s41698-023-00476-8. NPJ Precis Oncol. 2023. PMID: 38030881 Free PMC article. Review.
-
Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species.Proc Natl Acad Sci U S A. 2019 May 28;116(22):10943-10951. doi: 10.1073/pnas.1901777116. Epub 2019 May 16. Proc Natl Acad Sci U S A. 2019. PMID: 31097580 Free PMC article.
References
-
- Sheline GE, Wara WM, Smith M. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–28. - PubMed
-
- Central Brain Tumor Registry of the United States (CBTRUS) Primary brain and central nervous system tumors diagnosed in the United States. [updated 2010 Mar]. Available from: http://www.abta.org.
-
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol. 2002;63:129–45. - PubMed
-
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. - PubMed
-
- Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

