The human metabolic reconstruction Recon 1 directs hypotheses of novel human metabolic functions
- PMID: 21962087
- PMCID: PMC3224382
- DOI: 10.1186/1752-0509-5-155
The human metabolic reconstruction Recon 1 directs hypotheses of novel human metabolic functions
Abstract
Background: Metabolic network reconstructions formalize our knowledge of metabolism. Gaps in these networks pinpoint regions of metabolism where biological components and functions are "missing." At the same time, a major challenge in the post genomic era involves characterisation of missing biological components to complete genome annotation.
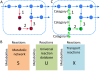
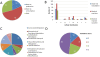
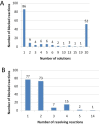
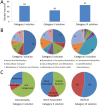
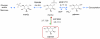
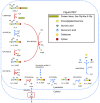
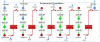
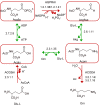
Results: We used the human metabolic network reconstruction RECON 1 and established constraint-based modelling tools to uncover novel functions associated with human metabolism. Flux variability analysis identified 175 gaps in RECON 1 in the form of blocked reactions. These gaps were unevenly distributed within metabolic pathways but primarily found in the cytosol and often caused by compounds whose metabolic fate, rather than production, is unknown. Using a published algorithm, we computed gap-filling solutions comprised of non-organism specific metabolic reactions capable of bridging the identified gaps. These candidate solutions were found to be dependent upon the reaction environment of the blocked reaction. Importantly, we showed that automatically generated solutions could produce biologically realistic hypotheses of novel human metabolic reactions such as of the fate of iduronic acid following glycan degradation and of N-acetylglutamate in amino acid metabolism.
Conclusions: The results demonstrate how metabolic models can be utilised to direct hypotheses of novel metabolic functions in human metabolism; a process that we find is heavily reliant upon manual curation and biochemical insight. The effectiveness of a systems approach for novel biochemical pathway discovery in mammals is demonstrated and steps required to tailor future gap filling algorithms to mammalian metabolic networks are proposed.
Figures
Similar articles
-
Inferring the metabolism of human orphan metabolites from their metabolic network context affirms human gluconokinase activity.Biochem J. 2013 Jan 15;449(2):427-35. doi: 10.1042/BJ20120980. Biochem J. 2013. PMID: 23067238
-
A detailed genome-wide reconstruction of mouse metabolism based on human Recon 1.BMC Syst Biol. 2010 Oct 19;4:140. doi: 10.1186/1752-0509-4-140. BMC Syst Biol. 2010. PMID: 20959003 Free PMC article.
-
A genome-scale, constraint-based approach to systems biology of human metabolism.Mol Biosyst. 2007 Sep;3(9):598-603. doi: 10.1039/b705597h. Epub 2007 Jul 11. Mol Biosyst. 2007. PMID: 17700859
-
Advances in gap-filling genome-scale metabolic models and model-driven experiments lead to novel metabolic discoveries.Curr Opin Biotechnol. 2018 Jun;51:103-108. doi: 10.1016/j.copbio.2017.12.012. Epub 2017 Dec 23. Curr Opin Biotechnol. 2018. PMID: 29278837 Review.
-
Systematizing the generation of missing metabolic knowledge.Biotechnol Bioeng. 2010 Oct 15;107(3):403-12. doi: 10.1002/bit.22844. Biotechnol Bioeng. 2010. PMID: 20589842 Free PMC article. Review.
Cited by
-
Plant Microbe Interactions in Post Genomic Era: Perspectives and Applications.Front Microbiol. 2016 Sep 26;7:1488. doi: 10.3389/fmicb.2016.01488. eCollection 2016. Front Microbiol. 2016. PMID: 27725809 Free PMC article. Review.
-
Systematic genomic analysis reveals the complementary aerobic and anaerobic respiration capacities of the human gut microbiota.Front Microbiol. 2014 Dec 5;5:674. doi: 10.3389/fmicb.2014.00674. eCollection 2014. Front Microbiol. 2014. PMID: 25538694 Free PMC article.
-
A conceptual review on systems biology in health and diseases: from biological networks to modern therapeutics.Syst Synth Biol. 2014 Mar;8(1):99-116. doi: 10.1007/s11693-013-9125-3. Epub 2013 Sep 18. Syst Synth Biol. 2014. PMID: 24592295 Free PMC article.
-
A community-driven global reconstruction of human metabolism.Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. Nat Biotechnol. 2013. PMID: 23455439 Free PMC article.
-
Global analysis of the human pathophenotypic similarity gene network merges disease module components.PLoS One. 2013;8(2):e56653. doi: 10.1371/journal.pone.0056653. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437198 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

