Quantitation of benzo[a]pyrene metabolic profiles in human bronchoalveolar (H358) cells by stable isotope dilution liquid chromatography-atmospheric pressure chemical ionization mass spectrometry
- PMID: 21962213
- PMCID: PMC3725129
- DOI: 10.1021/tx2002614
Quantitation of benzo[a]pyrene metabolic profiles in human bronchoalveolar (H358) cells by stable isotope dilution liquid chromatography-atmospheric pressure chemical ionization mass spectrometry
Abstract
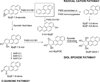
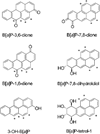
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants and are carcinogenic in multiple organs and species. Benzo[a]pyrene (B[a]P) is a representative PAH and has been studied extensively for its carcinogenicity and toxicity. B[a]P itself is chemically inert and requires metabolic activation to exhibit its toxicity and carcinogenicity. Three major metabolic pathways have been well documented. The signature metabolites generated from the radical cation (peroxidase or monooxygenase mediated) pathway are B[a]P-1,6-dione and B[a]P-3,6-dione, the signature metabolite generated from the diol-epoxide (P450 mediated) pathway is B[a]P-r-7,t-8,t-9,c-10-tetrahydrotetrol (B[a]P-tetrol-1), and the signature metabolite generated from the o-quinone (aldo-keto reductase mediated) pathway is B[a]P-7,8-dione. The contributions of these different metabolic pathways to cancer initiation and the exploitation of this information for cancer prevention are still under debate. With the availability of a library of [(13)C(4)]-labeled B[a]P metabolite internal standards, we developed a sensitive stable isotope dilution atmospheric pressure chemical ionization tandem mass spectrometry method to address this issue by quantitating B[a]P metabolites from each metabolic pathway in human lung cells. This analytical method represents a 500-fold increased sensitivity compared with that of a method using HPLC-radiometric detection. The limit of quantitation (LOQ) was determined to be 6 fmol on column for 3-hydroxybenzo[a]pyrene (3-OH-B[a]P), the generally accepted biomarker for B[a]P exposure. This high level of sensitivity and robustness of the method was demonstrated in a study of B[a]P metabolic profiles in human bronchoalveolar H358 cells induced or uninduced with the AhR ligand, 2,3,7,8-tetrachlorodibenzodioxin (TCDD). All the signature metabolites were detected and successfully quantitated. Our results suggest that all three metabolic pathways contribute equally in the overall metabolism of B[a]P in H358 cells with or without TCDD induction. The sensitivity of the method should permit the identification of cell-type differences in B[a]P activation and detoxication and could also be used for biomonitoring human exposure to PAH.
Figures



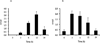



Similar articles
-
Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons.Chem Res Toxicol. 2014 Nov 17;27(11):1901-17. doi: 10.1021/tx500298n. Epub 2014 Oct 16. Chem Res Toxicol. 2014. PMID: 25279998 Free PMC article. Review.
-
Metabolism of benzo[a]pyrene in human bronchoalveolar H358 cells using liquid chromatography-mass spectrometry.Chem Res Toxicol. 2007 Sep;20(9):1331-41. doi: 10.1021/tx700107z. Epub 2007 Aug 17. Chem Res Toxicol. 2007. PMID: 17702526 Free PMC article.
-
Competing roles of cytochrome P450 1A1/1B1 and aldo-keto reductase 1A1 in the metabolic activation of (+/-)-7,8-dihydroxy-7,8-dihydro-benzo[a]pyrene in human bronchoalveolar cell extracts.Chem Res Toxicol. 2005 Feb;18(2):365-74. doi: 10.1021/tx0497245. Chem Res Toxicol. 2005. PMID: 15720144
-
Regulation of benzo[a]pyrene-mediated DNA- and glutathione-adduct formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human lung cells.Chem Res Toxicol. 2011 Jan 14;24(1):89-98. doi: 10.1021/tx100297z. Epub 2010 Oct 28. Chem Res Toxicol. 2011. PMID: 21028851 Free PMC article.
-
Dihydrodiol dehydrogenase and its role in polycyclic aromatic hydrocarbon metabolism.Chem Biol Interact. 1993 Oct;89(1):1-34. doi: 10.1016/0009-2797(93)03203-7. Chem Biol Interact. 1993. PMID: 8221964 Review.
Cited by
-
Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons.Chem Res Toxicol. 2014 Nov 17;27(11):1901-17. doi: 10.1021/tx500298n. Epub 2014 Oct 16. Chem Res Toxicol. 2014. PMID: 25279998 Free PMC article. Review.
-
Estrogen receptor-dependent and independent roles of benzo[a]pyrene in Ishikawa cells.J Endocrinol. 2020 Nov;247(2):139-151. doi: 10.1530/JOE-19-0579. J Endocrinol. 2020. PMID: 32992293 Free PMC article.
-
Relative quantification of biomarkers using mixed-isotope labeling coupled with MS.Bioanalysis. 2012 Oct;4(20):2525-41. doi: 10.4155/bio.12.208. Bioanalysis. 2012. PMID: 23157360 Free PMC article. Review.
-
The role of base excision repair genes OGG1, APN1 and APN2 in benzo[a]pyrene-7,8-dione induced p53 mutagenesis.Mutat Res. 2013 Jan 20;750(1-2):121-8. doi: 10.1016/j.mrgentox.2012.10.003. Epub 2012 Oct 29. Mutat Res. 2013. PMID: 23117049 Free PMC article.
-
Quantitation of enantiomers of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]-pyrene in human urine: evidence supporting metabolic activation of benzo[a]pyrene via the bay region diol epoxide.Mutagenesis. 2014 Sep;29(5):351-6. doi: 10.1093/mutage/geu024. Epub 2014 Jul 21. Mutagenesis. 2014. PMID: 25053834 Free PMC article.
References
-
- IARC-Monographs. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 32: Polynuclear Aromatic Compounds, Pt. 1: Chemical, Environmental and Experimental Data. 1983 - PubMed
-
- Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis (London) 1998;19:117–124. - PubMed
-
- Conney AH, Chang RL, Jerina DM, Wei SJC. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 1994;26:125–163. - PubMed
-
- Cavalieri EL, Rogan EG. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica. 1995;25:677–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

