Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes
- PMID: 21962237
- PMCID: PMC3203086
- DOI: 10.1186/1471-2172-12-55
Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes
Abstract
Background: Prolonged alcohol consumption is a significant co-factor in the progression of chronic viral infections including hepatitis C and HIV, which are both single-stranded RNA viruses. Toll like receptor 8 (TLR8), a pattern recognition receptor expressed in monocytes, senses viral single stranded RNA as a danger signal and leads to the induction of Type I interferon (IFN) as well as the pro-inflammatory cytokine, tumor necrosis factor alpha (TNF alpha). Lipopolysaccharide (LPS), a Toll like receptor 4 (TLR4) ligand, was shown to affect inflammatory cell activation after alcohol consumption and in HIV and HCV infections. Here we hypothesized that alcohol exposure modulates TLR8- and TLR4-ligand-induced monocyte activation and affects both type I IFN and inflammatory cytokine induction.
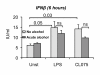
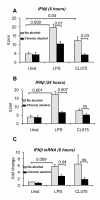
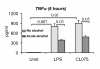
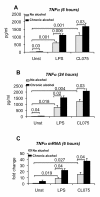
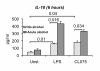
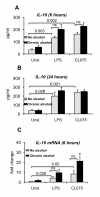
Results: The TLR8 ligand, CL075, as well as the TLR4 ligand, LPS, resulted in a significant induction of TNF alpha both at the mRNA and protein levels in human monocytes. We found that both acute and prolonged alcohol treatment resulted in inhibition of type I IFN induction by either TLR8 or TLR4 ligands in human monocytes at the protein and mRNA levels. In contrast to Type I IFN production, the effects of acute and prolonged alcohol were different on inflammatory cytokine activation after TLR8 or TLR4 ligand stimulation. Acute alcohol inhibited TLR8- or TLR4-induced TNF alpha protein and mRNA induction while it augmented IL-10 production in monocytes. In contrast, prolonged alcohol treatment augmented TNF alpha without affecting IL-10 production significantly in response to either TLR8 or TLR4 ligand stimulation.
Conclusions: These novel results suggest first, that alcohol has a profound inhibitory effect on Type I IFN induction regardless of intracellular (TLR8) or cell surface-derived (TLR4) danger signals. Second, both acute and prolonged alcohol exposure can inhibit antiviral Type I IFN pathway activation. Third, the opposite effects of acute (inhibitory) and prolonged alcohol (augmentation) treatment on pro-inflammatory cytokine activation extend to TLR8-induced signals beyond the previously shown TLR4/LPS pathway.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

