The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis
- PMID: 21962903
- PMCID: PMC3199340
- DOI: 10.1016/j.devcel.2011.08.019
The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis
Abstract
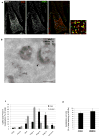
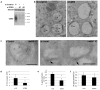
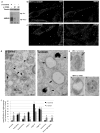
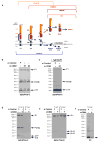
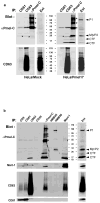
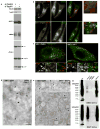
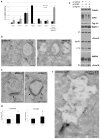
Cargo sorting to intraluminal vesicles (ILVs) of multivesicular endosomes is required for lysosome-related organelle (LRO) biogenesis. PMEL-a component of melanocyte LROs (melanosomes)-is sorted to ILVs in an ESCRT-independent manner, where it is proteolytically processed and assembled into functional amyloid fibrils during melanosome maturation. Here we show that the tetraspanin CD63 directly participates in ESCRT-independent sorting of the PMEL luminal domain, but not of traditional ESCRT-dependent cargoes, to ILVs. Inactivating CD63 in cell culture or in mice impairs amyloidogenesis and downstream melanosome morphogenesis. Whereas CD63 is required for normal PMEL luminal domain sorting, the disposal of the remaining PMEL transmembrane fragment requires functional ESCRTs but not CD63. In the absence of CD63, the PMEL luminal domain follows this fragment and is targeted for ESCRT-dependent degradation. Our data thus reveal a tight interplay regulated by CD63 between two distinct endosomal ILV sorting processes for a single cargo during LRO biogenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures

References
-
- Abache T, Le Naour F, Planchon S, Harper F, Boucheix C, Rubinstein E. The transferrin receptor and the tetraspanin web molecules CD9, CD81, and CD9P-1 are differentially sorted into exosomes after TPA treatment of K562 cells. J Cell Biochem. 2007;102:650–664. - PubMed
-
- Buschow SI, Nolte-‘t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. - PubMed
-
- Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420:133–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

