Angiotensin II mediates cell survival through upregulation and activation of the serum and glucocorticoid inducible kinase 1
- PMID: 21963429
- PMCID: PMC3237851
- DOI: 10.1016/j.cellsig.2011.09.016
Angiotensin II mediates cell survival through upregulation and activation of the serum and glucocorticoid inducible kinase 1
Abstract
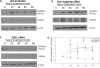
The serum- and glucocorticoid-inducible kinase 1 (SGK1) is known to regulate a wide variety of cellular processes, including renal sodium retention and cell survival. Angiotensin II (Ang II) is one of the many signaling molecules capable of regulating SGK1 expression, and is also known to impact cell survival. Here, we examined the role of SGK1 in Ang II-mediated cell survival. We hypothesized that Ang II protects cells from apoptosis by upregulating and activating SGK1. To test this, we examined the effects of Ang II stimulation on SGK1 expression and downstream signaling. We also examined the effects of Ang II treatment and siRNA-mediated SGK1 knockdown on apoptosis after serum starvation. We found that after 2h of Ang II treatment, SGK1 mRNA expression was increased approximately 2-fold. This induction was sensitive to reductions in intracellular calcium levels after pretreatment with BAPTA-AM, but insensitive to the L-type calcium channel blocker verapamil. SGK1 induction was also sensitive to the tyrosine kinase inhibitor genistein. Ang II treatment also caused a rapid increase in the level of phosphorylation of SGK1 at Ser422 and Thr256, and Ser422 phosphorylation was rapamycin-sensitive. We found that Ang II treatment was protective against serum starvation-induced apoptosis, and this protective effect was significantly blunted when SGK1 was silenced via siRNA. Lastly, Ang II induced FOXO3A phosphorylation in an SGK1-dependent manner, thereby reducing the pro-apoptotic actions of FOXO3A. Overall, these results indicate that Ang II upregulates and activates SGK1, leading to increased cell survival via multiple, non-redundant mechanisms.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures







References
-
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. Physiol Rev. 2006;86:1151–1178. - PubMed
-
- Zhang L, Cui R, Cheng X, Du J. Cancer Res. 2005;65:457–464. - PubMed
-
- Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Cancer Res. 2004;64:1757–1764. - PubMed
-
- Mehta PK, Griendling KK. Am J Physiol Cell Physiol. 2007;292:C82–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

