Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis
- PMID: 21963537
- PMCID: PMC3215829
- DOI: 10.1016/j.ydbio.2011.09.014
Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis
Abstract
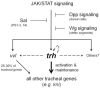
The Drosophila trachea is a branched tubular epithelia that transports oxygen and other gases. trachealess (trh), which encodes a bHLH-PAS transcription factor, is among the first genes to be expressed in the cells that will form the trachea. In the absence of trh, tracheal cells fail to invaginate to form tubes and remain on the embryo surface. Expression of many tracheal-specific genes depends on trh, but all of the known targets have relatively minor phenotypes compared to loss of trh, suggesting that there are additional targets. To identify uncharacterized transcriptional targets of Trh and to further understand the role of Trh in embryonic tracheal formation, we performed an in situ hybridization screen using a library of ~100 tracheal-expressed genes identified by the Berkeley Drosophila Genome Project (BDGP). Surprisingly, expression of every tracheal gene we tested was dependent on Trh, suggesting a major role for Trh in activation and maintenance of tracheal gene expression. A re-examination of the interdependence of the known early-expressed transcription factors, including trh, ventral veinless (vvl) and knirps/knirps-related (kni/knrl), suggests a new model for how gene expression is controlled in the trachea, with trh regulating expression of vvl and kni, but not vice versa. A pilot screen for the targets of Vvl and Kni/Knrl revealed that Vvl and Kni have only minor roles compared to Trh. Finally, genome-wide microarray experiments identified additional Trh targets and revealed that a variety of biological processes are affected by the loss of trh.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

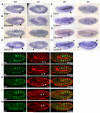
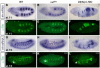
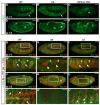
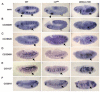
Similar articles
-
The Drosophila jing gene is a downstream target in the Trachealess/Tango tracheal pathway.Dev Genes Evol. 2010 Dec;220(7-8):191-206. doi: 10.1007/s00427-010-0339-z. Dev Genes Evol. 2010. PMID: 21061019
-
Transcriptional control of steroid biosynthesis genes in the Drosophila prothoracic gland by ventral veins lacking and knirps.PLoS Genet. 2014 Jun 19;10(6):e1004343. doi: 10.1371/journal.pgen.1004343. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24945799 Free PMC article.
-
ventral veinless, a POU domain transcription factor, regulates different transduction pathways required for tracheal branching in Drosophila.Development. 1997 Sep;124(17):3273-81. doi: 10.1242/dev.124.17.3273. Development. 1997. PMID: 9310322
-
The Drosophila dysfusion basic helix-loop-helix (bHLH)-PAS gene controls tracheal fusion and levels of the trachealess bHLH-PAS protein.Mol Cell Biol. 2003 Aug;23(16):5625-37. doi: 10.1128/MCB.23.16.5625-5637.2003. Mol Cell Biol. 2003. PMID: 12897136 Free PMC article.
-
An efficient approach to isolate STAT regulated enhancers uncovers STAT92E fundamental role in Drosophila tracheal development.Dev Biol. 2010 Apr 15;340(2):571-82. doi: 10.1016/j.ydbio.2010.02.015. Epub 2010 Feb 18. Dev Biol. 2010. PMID: 20171201 Free PMC article.
Cited by
-
Mechanisms of Na+ uptake from freshwater habitats in animals.Front Physiol. 2022 Oct 18;13:1006113. doi: 10.3389/fphys.2022.1006113. eCollection 2022. Front Physiol. 2022. PMID: 36388090 Free PMC article. Review.
-
A comparison of midline and tracheal gene regulation during Drosophila development.PLoS One. 2014 Jan 20;9(1):e85518. doi: 10.1371/journal.pone.0085518. eCollection 2014. PLoS One. 2014. PMID: 24465586 Free PMC article.
-
Blimp-1 Mediates Tracheal Lumen Maturation in Drosophila melanogaster.Genetics. 2018 Oct;210(2):653-663. doi: 10.1534/genetics.118.301444. Epub 2018 Aug 6. Genetics. 2018. PMID: 30082278 Free PMC article.
-
The Drosophila Zinc Finger Transcription Factor Ouija Board Controls Ecdysteroid Biosynthesis through Specific Regulation of spookier.PLoS Genet. 2015 Dec 10;11(12):e1005712. doi: 10.1371/journal.pgen.1005712. eCollection 2015 Dec. PLoS Genet. 2015. PMID: 26658797 Free PMC article.
-
The dynamics of tubulogenesis in development and disease.Development. 2025 Feb 1;152(3):DEV202820. doi: 10.1242/dev.202820. Epub 2025 Feb 17. Development. 2025. PMID: 39959988 Review.
References
-
- Abrams EW, Andrew DJ. CrebA regulates secretory activity in the Drosophila salivary gland and epidermis. Development. 2005;132:2743–2758. - PubMed
-
- Anderson MG, Certel SJ, Certel K, Lee T, Montell DJ, Johnson WA. Function of the Drosophila POU domain transcription factor drifter as an upstream regulator of breathless receptor tyrosine kinase expression in developing trachea. Development. 1996;122:4169–4178. - PubMed
-
- Anderson MG, Perkins GL, Chittick P, Shrigley RJ, Johnson WA. drifter, a Drosophila POU-domain transcription factor, is required for correct differentiation and migration of tracheal cells and midline glia. Genes Dev. 1995;9:123–137. - PubMed
-
- Boube M, Llimargas M, Casanova J. Cross-regulatory interactions among tracheal genes support a co-operative model for the induction of tracheal fates in the Drosophila embryo. Mech Dev. 2000;91:271–278. - PubMed
-
- Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–1705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

