PET imaging for attention deficit preclinical drug testing in neurofibromatosis-1 mice
- PMID: 21963652
- PMCID: PMC3202049
- DOI: 10.1016/j.expneurol.2011.09.005
PET imaging for attention deficit preclinical drug testing in neurofibromatosis-1 mice
Abstract
Attention system abnormalities represent a significant barrier to scholastic achievement in children with neurofibromatosis-1 (NF1). Using a novel mouse model of NF1-associated attention deficit (ADD), we demonstrate a presynaptic defect in striatal dopaminergic homeostasis and leverage this finding to apply [(11)C]-raclopride positron-emission tomography (PET) in the intact animal. While methylphenidate and l-Deprenyl correct both striatal dopamine levels on PET imaging and defective attention system function in Nf1 mutant mice, pharmacologic agents that target de-regulated cyclic AMP and RAS signaling in these mice do not. These studies establish a robust preclinical model to evaluate promising agents for NF1-associated ADD.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
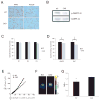
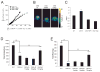
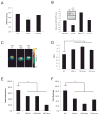
References
-
- Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. - PubMed
-
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. - PubMed
-
- Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–2760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

