Accuracy and resolution of in vitro imaging based porcine lens volumetric measurements
- PMID: 21963717
- PMCID: PMC3658159
- DOI: 10.1016/j.exer.2011.09.008
Accuracy and resolution of in vitro imaging based porcine lens volumetric measurements
Abstract
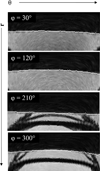
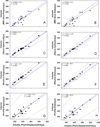
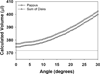
There is considerable interest in determining lens volume in the living eye. Lens volume is of interest to understand accommodative changes in the lens and to size accommodative IOLs (A-IOLs) to fit the capsular bag. Some studies have suggested lens volume change during accommodation. Magnetic Resonance Imaging (MRI) is the only method available to determine lens volume in vivo. MRI is, by its nature, relatively low in temporal and spatial resolution. Therefore analysis often requires determining lens volume from single image slices with relatively low resolution on which only simple image analysis methods can be used and without repeated measures. In this study, 7 T MRI scans encompassing the full lens volume were performed on 19 enucleated pig eyes. The eyes were then dissected to isolate and photograph the lens in profile and the lens volumes were measured empirically using a fluid displacement method. Lens volumes were calculated from two- and three-dimensional (2D and 3D) MR and 2D photographic profile images of the isolated lenses using several different analysis methods. Image based and actual measured lens volumes were compared. The average image-based volume of all lenses varied from the average measured volume of all lenses by 0.6%-6.4% depending on the image analysis method. Image analysis methods that use gradient based edge detection showed higher precision with actual volumes (r(2): 0.957-0.990), while threshold based segmentation had poorer correlations (r(2): 0.759-0.828). The root-mean-square (RMS) difference between image analysis based volumes and fluid displacement measured volumes ranged from 8.51 μl to 25.79 μl. This provides an estimate of the error of previously published methods used to calculate lens volume. Immobilized, enucleated porcine eyes permit improved MR image resolution relative to living eyes and therefore improved image analysis methods to calculate lens volume. The results show that some of the accommodative changes in lens volume reported in the literature are likely below the resolution limits of imaging methods used. MRI, even with detailed image analysis methods used here, is unlikely to achieve the resolution required to accurately size an A-IOL to the capsular bag.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures






References
-
- Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008;8 29-20. - PubMed
-
- Baikoff G, Lutun E, Wei J, Ferraz C. Anterior chamber optical coherence tomography study of human natural accommodation in a 19-year-old albino. J. Cataract Refract. Surg. 2004;30:696–701. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

