Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair
- PMID: 21963833
- PMCID: PMC3233810
- DOI: 10.1152/ajpheart.00343.2011
Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair
Abstract
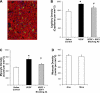
The skeletal muscle is endowed with an impressive ability to regenerate after injury, and this ability is coupled to paracrine production of many trophic factors possessing cardiovascular benefits. Taking advantage of this humoral capacity of the muscle, we recently demonstrated an extracardiac therapeutic regimen based on intramuscular delivery of VEGF-A(165) for repair of the failing hamster heart. This distal organ repair mechanism activates production from the injected hamstring of many trophic factors, among which stromal-derived factor-1 (SDF1) prominently mobilized multi-lineage progenitor cells expressing CXCR4 and their recruitment to the heart. The mobilized bone marrow progenitor cells express the cardiac transcription factors myocyte enhancer factor 2c and GATA4 and several major trophic factors, most notably IGF1 and VEGF. SDF1 blockade abrogated myocardial recruitment of CXCR4(+) and c-kit(+) progenitor cells with an insignificant effect on the hematopoietic progenitor lineage. The knockdown of cardiac progenitor cells led to deprivation of myocardial trophic factors, resulting in compromised cardiomyogenesis and angiogenesis. However, the VEGF-injected hamstring continued to synthesize cardioprotective factors, contributing to moderate myocardial tissue viability and function even in the presence of SDF1 blockade. These findings thus uncover two distinct but synergistic cardiac therapeutic mechanisms activated by intramuscular VEGF. Whereas the SDF1/CXCR4 axis activates the progenitor cell cascade and its trophic support of cardiomyogenesis intramuscularly, VEGF amplifies the skeletal muscle paracrine cascade capable of directly promoting myocardial survival independent of SDF1. Given that recent clinical trials of cardiac repair based on the use of marrow-mobilizing agents have been disappointing, the proposed dual therapeutic modality warrants further investigation.
Figures




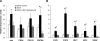

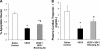



References
-
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110: 3300–3305, 2004 - PubMed
-
- Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther 10: 844–854, 2004 - PubMed
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA 104: 14068–14073, 2007 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

