A selective phosphodiesterase 3 inhibitor rescues low PO2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control
- PMID: 21963837
- PMCID: PMC3233804
- DOI: 10.1152/ajpheart.00729.2011
A selective phosphodiesterase 3 inhibitor rescues low PO2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control
Erratum in
- Am J Physiol Heart Circ Physiol. 2012 Jan;302(1):H378
Abstract
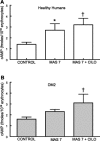
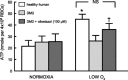
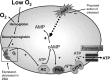
Erythrocytes, via release of ATP in areas of low oxygen (O(2)) tension, are components of a regulatory system for the distribution of perfusion in skeletal muscle ensuring optimal O(2) delivery to meet tissue needs. In type 2 diabetes (DM2), there are defects in O(2) supply to muscle as well as a failure of erythrocytes to release ATP. The goal of this study was to ascertain if a phosphodiesterase 3 (PDE3) inhibitor, cilostazol, would rescue low O(2)-induced ATP release from DM2 erythrocytes and, thereby, enable these cells to dilate isolated erythrocyte-perfused skeletal muscle arterioles exposed to decreased extraluminal O(2). Erythrocytes were obtained from healthy humans (HH; n = 12) and humans with DM2 (n = 17). We determined that 1) PDE3B is similarly expressed in both groups, 2) mastoparan 7 (G(i) activation) stimulates increases in cAMP in HH but not in DM2 erythrocytes, and 3) pretreatment of DM2 erythrocytes with cilostazol resulted in mastoparan 7-induced increases in cAMP not different from those in HH cells. Most importantly, cilostazol restored the ability of DM2 erythrocytes to release ATP in response to low O(2). In contrast with perfusion with HH erythrocytes, isolated hamster retractor muscle arterioles perfused with DM2 erythrocytes constricted in response to low extraluminal PO(2). However, in the presence of cilostazol (100 μM), DM2 erythrocytes induced vessel dilation not different from that seen with HH erythrocytes. Thus rescue of low O(2)-induced ATP release from DM2 erythrocytes by cilostazol restored the ability of erythrocytes to participate in the regulation of perfusion distribution in skeletal muscle.
Figures


References
-
- Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 285: H1404–H1410, 2003 - PubMed
-
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnea. Cardiovasc Res 26: 40–47, 1992 - PubMed
-
- Chalmers C, Bird BD, Whitwam JG. Evaluation of a new thin film tonometer. Br J Anaesth 46: 253–259, 1974 - PubMed
-
- Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvas Res 56: 43–53, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

