Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins
- PMID: 21963839
- PMCID: PMC3233808
- DOI: 10.1152/ajpheart.00503.2011
Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins
Abstract
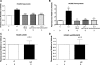
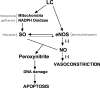
Light chain amyloidosis (AL) involves overproduction of amyloidogenic light chain proteins (LC) leading to heart failure, yet the mechanisms underlying tissue toxicity remain unknown. We hypothesized that LC induces endothelial dysfunction in non-AL human microvasculature and apoptotic injury in human coronary artery endothelial cells (HCAECs). Adipose arterioles (n = 34, 50 ± 3 yr) and atrial coronary arterioles (n = 19, 68 ± 2 yr) from non-AL subjects were cannulated. Adipose arteriole dilator responses to acetylcholine/papaverine were measured at baseline and 1 h exposure to LC (20 μg/ml) from biopsy-proven AL subjects (57 ± 11 yr) without and with antioxidant cotreatment. Coronary arteriole dilation to bradykinin/papaverine was measured post-LC exposure. HCAECs were exposed to 1 or 24 h of LC. LC reduced dilation to acetylcholine (10(-4) M: 41.6 ± 7 vs. 85.8 ± 2.2% control, P < 0.001) and papaverine (81.4 ± 4.6 vs. 94.8 ± 1.3% control, P < 0.01) in adipose arterioles and to bradykinin (10(-6) M: 68.6 ± 6.2 vs. 90.9 ± 1.6% control, P < 0.001) but not papaverine in coronary arterioles. There was an increase in superoxide and peroxynitrite in arterioles treated with LC. Adipose arteriole dilation was restored by cotreatment with polyethylene glycol-superoxide dismutase and tetrahydrobiopterin but only partially restored by mitoquinone (mitochondria-targeted antioxidant) and gp91ds-tat (NADPH oxidase inhibitor). HCAECs exposed to LC showed reduced NO and increased superoxide, peroxynitrite, annexin-V, and propidium iodide compared with control. Brief exposure to physiological amounts of LC induced endothelial dysfunction in human adipose and coronary arterioles and increased apoptotic injury in coronary artery endothelial cells likely as a result of oxidative stress, reduced NO bioavailability, and peroxynitrite production. Microvascular dysfunction and injury is a novel mechanism underlying AL pathobiology and is a potential target for therapy.
Figures



References
-
- Ando Y, Nyhlin N, Suhr O, Holmgren G, Uchida K, el Sahly M, Yamashita T, Terasaki H, Nakamura M, Uchino M, Ando M. Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochem Biophys Res Commun 232: 497–502, 1997 - PubMed
-
- Ando Y, Suhr O, el-Salhy M. Oxidative stress and amyloidosis. Histol Histopathol 13: 845–850, 1998 - PubMed
-
- Bellavia D, Pellikka PA, Al-Zahrani G, Abraham TP, Dispenzieri A, Miyazaki C, Lacy MQ, Scott CG, Oh JK, Miller FA. Independent predictors of survival in primary (AL) amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr 23: 643–652, 2010 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

