EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation
- PMID: 21963850
- PMCID: PMC3711644
- DOI: 10.1038/onc.2011.450
EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation
Abstract
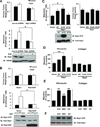
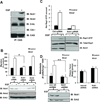
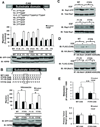
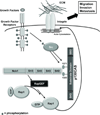
Tyrosine kinase receptors have an essential role in various aspects of tumor progression. In particular, epidermal growth factor receptor (EGFR) and its ligands have been implicated in the growth and dissemination of a wide array of human carcinomas. Here, we describe an EGFR-mediated signaling pathway that regulates human pancreatic carcinoma cell invasion and metastasis, yet does not influence the growth of primary tumors. In fact, ligation/activation of EGFR induces Src-dependent phosphorylation of two critical tyrosine residues of p130CAS, leading to the assembly of a Crk-associated substrate (CAS)/Nck1 complex that promotes Ras-associated protein-1 (Rap1) signaling. Importantly, GTP loading of Rap1 is specifically required for pancreatic carcinoma cell migration on vitronectin but not on collagen. Furthermore, Rap1 activation is required for EGFR-mediated metastasis in vivo without impacting primary tumor growth. These findings identify a molecular pathway that promotes the invasive/metastatic properties of human pancreatic carcinomas driven by EGFR.
Conflict of interest statement
Figures

References
-
- Bos JL. Linking Rap to cell adhesion. Current Opinion in Cell Biology. 2005;17:123–128. - PubMed
-
- Brabek J, Constancio SS, Shin NY, Pozzi A, Weaver AM, Hanks SK. CAS promotes invasiveness of Src-transformed cells. Oncogene. 2004;23:7406–7415. - PubMed
-
- Brabek J, Constancio SS, Siesser PF, Shin NY, Pozzi A, Hanks SK. Crk-Associated Substrate Tyrosine Phosphorylation Sites Are Critical for Invasion and Metastasis of Src-Transformed Cells. Mol Cancer Res. 2005;3:307–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

