Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans
- PMID: 21964028
- PMCID: PMC3359935
- DOI: 10.4049/jimmunol.1101000
Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans
Abstract
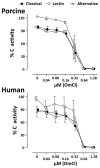
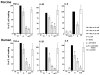
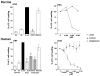
Experimental evidence suggests that C inhibition and more particularly combined inhibition of C and the TLR coreceptor CD14 may be of therapeutic benefit in sepsis and other inflammatory conditions. A barrier to the testing and further development of many inhibitors is that their activity is species specific. Pig is a relevant species for experimental models of human disease, and this study undertakes a comprehensive comparison of the inhibitory efficacy of the C5 inhibitor Ornithodoros moubata C inhibitor (OmCI) in human and porcine whole blood ex vivo models of Escherichia coli-induced sepsis. The effect of OmCI on complement activity in pigs undergoing E. coli sepsis was also examined. Porcine and human serum, and whole blood anticoagulated with lepirudin, was incubated with E. coli and the effect of OmCI investigated. The ex vivo results were virtually identical in pig and human. OmCI completely ablated the activity of all three C pathways at 0.64 μM. E. coli-induced C activation and expression of CD11b (wCD11R3 in the pig), was abolished ex vivo at 0.32 μM OmCI. Combining anti-CD14 and OmCI reduced the formation of IL-8 and TNF-α more potently than the single inhibitors. OmCI also efficiently bound E. coli-induced leukotriene B(4) in pig and human plasma. In support of our ex vivo findings, in vivo the activity of all C pathways was inhibited at 0.6 mg OmCI/kg pig. In conclusion, OmCI efficiently inhibited pig and human C activation, has accompanying anti-inflammatory effects and is a promising candidate inhibitor for further in vivo studies of sepsis.
Figures


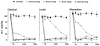
References
-
- Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. - PubMed
-
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. - PubMed
-
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. - PubMed
-
- Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

