Protor-2 interacts with tristetraprolin to regulate mRNA stability during stress
- PMID: 21964062
- PMCID: PMC3205320
- DOI: 10.1016/j.cellsig.2011.09.015
Protor-2 interacts with tristetraprolin to regulate mRNA stability during stress
Abstract
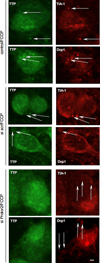
The A/U-rich RNA-binding protein tristetraprolin (TTP) is an mRNA destabilizing factor which plays a role in the regulated turnover of many transcripts encoding proteins involved in immune function and cell growth control. TTP also plays a role in stress-induced destabilization of mRNAs. Here we report the interaction of TTP with a component of the mTORC2 kinase, Protor-2 (PRR5-L, protein Q6MZQ0/FLJ14213/CAE45978). Protor-2 is structurally similar to human PRR5 and has been demonstrated to bind mTORC2 via Rictor and/or Sin1 and may signal downstream events promoting apoptosis. Protor-2 dissociates from mTORC2 upon hyperactivation of the kinase and is not required for mTORC2 integrity or activity. We identified Protor-2 in a yeast two-hybrid screen as a TTP interactor using the C-terminal mRNA decay domain of TTP as bait. The interaction of Protor-2 with TTP was also confirmed in vivo in co-immunoprecipitation experiments and Protor-2 was also detected in immunoprecipitates of Rictor. Protor-2 was shown to stimulate TTP-mediated mRNA turnover of several TTP-associated mRNAs (TNF-α, GM-CSF, IL-3 and COX-2) in Jurkat cells when overexpressed while the half-lives of transcripts which do not decay via a TTP-mediated mechanism were unaffected. Knockdown of Protor-2 via RNAi inhibited TTP-mediated mRNA turnover of these TTP-associated mRNAs and inhibited association of TTP with cytoplasmic stress granules (SG) or mRNA processing bodies (P-bodies) following induction of the integrated stress response. These results suggest that Protor-2 associates with TTP to accelerate TTP-mediated mRNA turnover and functionally links the control of TTP-regulated mRNA stability to mTORC2 activity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

