Akt2 knockout mitigates chronic iNOS inhibition-induced cardiomyocyte atrophy and contractile dysfunction despite persistent insulin resistance
- PMID: 21964073
- PMCID: PMC3208377
- DOI: 10.1016/j.toxlet.2011.09.015
Akt2 knockout mitigates chronic iNOS inhibition-induced cardiomyocyte atrophy and contractile dysfunction despite persistent insulin resistance
Erratum in
- Toxicol Lett. 2016 Apr 15;247():71-2
Abstract
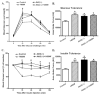
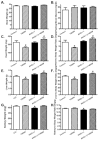
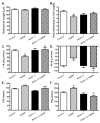
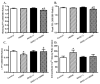
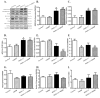
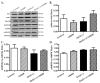
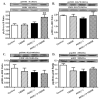
Increased levels of inducible nitric oxide synthase (iNOS) during cardiac stress such as ischemia-reperfusion, sepsis and hypertension may display both beneficial and detrimental roles in cardiac contractile performance. However, the precise role of iNOS in the maintenance of cardiac contractile function remains elusive. This study was designed to determine the impact of chronic iNOS inhibition on cardiac contractile function and the underlying mechanism involved with a special focus on the NO downstream signaling molecule Akt. Male C57 or Akt2 knockout [Akt2(-/-)] mice were injected with the specific iNOS inhibitor 1400W (2 mg/kg/d) or saline for 7 days. Both 1400W and Akt2 knockout dampened glucose and insulin tolerance without additive effects. Treatment of 1400W decreased heart and liver weights as well as cardiomyocyte cross-sectional area in C57 but not Akt2 knockout mice. 1400W but not Akt2 knockout compromised cardiomyocyte mechanical properties including decreased peak shortening and maximal velocity of shortening/relengthening, prolonged relengthening duration, reduced intracellular Ca(2+) release and decay rate, the effects of which were ablated or attenuated by Akt2 knockout. Akt2 knockout but not 1400W increased the levels of intracellular Ca(2+) regulatory proteins including SERCA2a and phospholamban phosphorylation. 1400W reduced the level of anti-apoptotic protein Bcl-2, the effect of which was unaffected by Akt2 knockout. Neither 1400W nor Akt2 knockout significantly affected ER stress, autophagy, the post-insulin receptor signaling Akt, GSK3β and AMPK, as well as the stress signaling IκB, JNK, ERK and p38 with the exception of elevated IκB phosphorylation with jointed effect of 1400W and Akt2 knockout. Taken together, these data indicated that an essential role of iNOS in the maintenance of cardiac morphology and function possibly through an Akt2-dependent mechanism.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Bai-Feng L, Yong-Feng L, Ying C. Silencing inducible nitric oxide synthase protects rat pancreatic islet. Diabetes Res Clin Pract. 2010;89:268–275. - PubMed
-
- Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. - PubMed
-
- Ceriello A, Quagliaro L, D’Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51:1076–1082. - PubMed
-
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. - PubMed
-
- De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev. 2010;15:423–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

