MAP0004, orally inhaled dihydroergotamine for acute treatment of migraine: efficacy of early and late treatments
- PMID: 21964172
- PMCID: PMC3184024
- DOI: 10.4065/mcp.2011.0093
MAP0004, orally inhaled dihydroergotamine for acute treatment of migraine: efficacy of early and late treatments
Erratum in
- Mayo Clin Proc. 2011 Dec;86(12):1248
Abstract
Objective: To evaluate the efficacy of MAP0004, an orally inhaled dihydroergotamine, for acute treatment of migraine when administered at various time points from within 1 hour to more than 8 hours after migraine onset.
Patients and methods: This post hoc subanalysis was conducted using data from 902 patients enrolled in a randomized, double-blind, placebo-controlled, 2-arm, phase 3, multicenter study conducted from July 14, 2008, through March 23, 2009. End points were 2-hour pain relief and pain-free rates in patients who treated a migraine in ≤1 hour, from >1 hour to ≤4 hours, from >4 to ≤8 hours, or in >8 hours after onset of migraine, given that patients may be unwilling or unable to initiate treatment at headache inception.
Results: Treatment with MAP0004 was significantly more effective than placebo in relieving pain at all treatment points (≤1 hour after start of migraine: 66% [74/112] for MAP0004 vs 41% [48/118] for placebo, P<.001; >1 to ≤4 hours: 60% [91/153] vs 35% [58/168], P<.001; >4 to ≤8 hours: 53% [36/68] vs 30% [16/54], P=.008; and >8 hours: 48% [25/52] vs 24% [11/46], P=.007). Pain-free rates were also significantly higher with MAP0004 than placebo for treatment within 8 hours after migraine onset (≤1 hour: 38% [43/112] for MAP0004 vs 13% [15/118] for placebo, P<.001; >1 to ≤4 hours: 28% [43/153] vs 10% [17/168], P<.001; >4 to ≤8 hours: 22% [15/68] vs 7% [4/54], P<.025) but not at >8 hours (19% [10/52] vs 9% [4/46], P=.106).
Conclusion: This post hoc subanalysis shows that MAP0004 was effective in treating migraine irrespective of the time of treatment, even more than 8 hours after onset of migraine pain.
Trial registration: ClinicalTrials.gov NCT00623636.
Figures

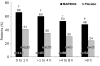
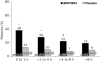
References
-
- Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193-210 - PubMed
-
- Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition Cephalalgia. 2004;24(suppl 1):9-160 - PubMed
-
- Valade D. Early treatment of acute migraine: new evidence of benefits. Cephalalgia. 2009;29(suppl 3):15-21 - PubMed
-
- Cady R, Elkind A, Goldstein J, Keywood C. Randomized, placebo-controlled comparison of early use of frovatriptan in a migraine attack versus dosing after the headache has become moderate or severe. Curr Med Res Opin. 2004;20(9):1465-1472 - PubMed
-
- Cady R, Martin V, Mauskop A, et al. Efficacy of rizatriptan 10 mg administered early in a migraine attack. Headache. 2006;46(6):914-924 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

