Crystal structure of the passenger domain of the Escherichia coli autotransporter EspP
- PMID: 21964244
- PMCID: PMC3215816
- DOI: 10.1016/j.jmb.2011.09.028
Crystal structure of the passenger domain of the Escherichia coli autotransporter EspP
Abstract
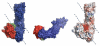
Autotransporters represent a large superfamily of known and putative virulence factors produced by Gram-negative bacteria. They consist of an N-terminal "passenger domain" responsible for the specific effector functions of the molecule and a C-terminal "β-domain" responsible for translocation of the passenger across the bacterial outer membrane. Here, we present the 2.5-Å crystal structure of the passenger domain of the extracellular serine protease EspP, produced by the pathogen Escherichia coli O157:H7 and a member of the serine protease autotransporters of Enterobacteriaceae (SPATEs). Like the previously structurally characterized SPATE passenger domains, the EspP passenger domain contains an extended right-handed parallel β-helix preceded by an N-terminal globular domain housing the catalytic function of the protease. Of note, however, is the absence of a second globular domain protruding from this β-helix. We describe the structure of the EspP passenger domain in the context of previous results and provide an alternative hypothesis for the function of the β-helix within SPATEs.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





References
-
- Dautin N, Bernstein HD. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol. 2007;61:89–112. - PubMed
-
- Jose J, Jähnig F, Meyer TF. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol. 1995;18:378–380. - PubMed
-
- Pohlner J, Halter R, Beyreuther K, Meyer T. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. - PubMed
-
- Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

