Anthrax sub-unit vaccine: the structural consequences of binding rPA83 to Alhydrogel®
- PMID: 21964315
- PMCID: PMC3244517
- DOI: 10.1016/j.ejpb.2011.09.009
Anthrax sub-unit vaccine: the structural consequences of binding rPA83 to Alhydrogel®
Abstract
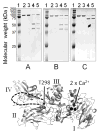
An anthrax sub-unit vaccine, comprising recombinant Protective Antigen (rPA83) and aluminium hydroxide adjuvant (Alhydrogel®) is currently being developed. Here, a series of biophysical techniques have been applied to free and adjuvant bound antigen. Limited proteolysis and fluorescence identified no changes in rPA83 tertiary structure following binding to Alhydrogel and the bound rPA83 retained two structurally important calcium ions. For adsorbed rPA83, differential scanning calorimetry revealed a small reduction in unfolding temperature but a large decrease in unfolding enthalpy whilst urea unfolding demonstrated unchanged stability but a loss of co-operativity. Overall, these results demonstrate that interactions between rPA83 and Alhydrogel have a minimal effect on the folded protein structure and suggest that antigen destabilisation is not a primary mechanism of Alhydrogel adjuvancy. This study also shows that informative structural characterisation is possible for adjuvant bound sub-unit vaccines.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Structural and immunological analysis of anthrax recombinant protective antigen adsorbed to aluminum hydroxide adjuvant.Clin Vaccine Immunol. 2012 Sep;19(9):1465-73. doi: 10.1128/CVI.00174-12. Epub 2012 Jul 18. Clin Vaccine Immunol. 2012. PMID: 22815152 Free PMC article.
-
Evaluation of the compatibility of a second generation recombinant anthrax vaccine with aluminum-containing adjuvants.Vaccine. 2003 Jun 20;21(21-22):3011-8. doi: 10.1016/s0264-410x(03)00109-9. Vaccine. 2003. PMID: 12798645
-
Increasing the potency of an alhydrogel-formulated anthrax vaccine by minimizing antigen-adjuvant interactions.Clin Vaccine Immunol. 2013 Nov;20(11):1659-68. doi: 10.1128/CVI.00320-13. Epub 2013 Aug 28. Clin Vaccine Immunol. 2013. PMID: 23986317 Free PMC article.
-
Effect of aluminum hydroxide adjuvant and formaldehyde in the formulation of rPA anthrax vaccine.Vaccine. 2007 Apr 12;25(15):2771-7. doi: 10.1016/j.vaccine.2006.12.043. Epub 2007 Jan 2. Vaccine. 2007. PMID: 17240008
-
Freeze-thaw stress of Alhydrogel ® alone is sufficient to reduce the immunogenicity of a recombinant hepatitis B vaccine containing native antigen.Vaccine. 2014 Jun 24;32(30):3765-71. doi: 10.1016/j.vaccine.2014.05.037. Epub 2014 May 20. Vaccine. 2014. PMID: 24856785
Cited by
-
Helix N-Cap Residues Drive the Acid Unfolding That Is Essential in the Action of the Toxin Colicin A.Biochemistry. 2019 Dec 3;58(48):4882-4892. doi: 10.1021/acs.biochem.9b00705. Epub 2019 Nov 13. Biochemistry. 2019. PMID: 31686499 Free PMC article.
-
Structural and immunological analysis of anthrax recombinant protective antigen adsorbed to aluminum hydroxide adjuvant.Clin Vaccine Immunol. 2012 Sep;19(9):1465-73. doi: 10.1128/CVI.00174-12. Epub 2012 Jul 18. Clin Vaccine Immunol. 2012. PMID: 22815152 Free PMC article.
-
Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes.Clin Exp Vaccine Res. 2015 Jan;4(1):23-45. doi: 10.7774/cevr.2015.4.1.23. Epub 2015 Jan 30. Clin Exp Vaccine Res. 2015. PMID: 25648619 Free PMC article. Review.
-
Aluminum Adjuvants-'Back to the Future'.Pharmaceutics. 2023 Jul 4;15(7):1884. doi: 10.3390/pharmaceutics15071884. Pharmaceutics. 2023. PMID: 37514070 Free PMC article. Review.
-
Mechanism of immunopotentiation and safety of aluminum adjuvants.Front Immunol. 2013 Jan 10;3:406. doi: 10.3389/fimmu.2012.00406. eCollection 2012. Front Immunol. 2013. PMID: 23335921 Free PMC article.
References
-
- Turnbull PCB. Anthrax Vaccines - Past. Present and Future Vaccine. 1991;9:533–539. - PubMed
-
- Liljeqvist S, Stahl S. Production of recombinant subunit vaccines:protein immunogens, live delivery systems and nucleic acid vaccines. Journal of Biotechnology. 1999;73:1–33. - PubMed
-
- O’Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomolecular Engineering. 2001;18:69–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

