Structure determination of membrane proteins in five easy pieces
- PMID: 21964394
- PMCID: PMC3264820
- DOI: 10.1016/j.ymeth.2011.09.009
Structure determination of membrane proteins in five easy pieces
Abstract
Rotational Alignment (RA) solid-state NMR provides the basis for a general method for determining the structures of membrane proteins in phospholipid bilayers under physiological conditions. Membrane proteins are high priority targets for structure determination, and are challenging for existing experimental methods. Because membrane proteins reside in liquid crystalline phospholipid bilayer membranes it is important to study them in this type of environment. The RA solid-state NMR approach we have developed can be summarized in five steps, and incorporates methods of molecular biology, biochemistry, sample preparation, the implementation of NMR experiments, and structure calculations. It relies on solid-state NMR spectroscopy to obtain high-resolution spectra and residue-specific structural restraints for membrane proteins that undergo rotational diffusion around the membrane normal, but whose mobility is otherwise restricted by interactions with the membrane phospholipids. High resolution spectra of membrane proteins alone and in complex with other proteins and ligands set the stage for structure determination and functional studies of these proteins in their native, functional environment.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



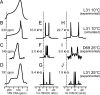
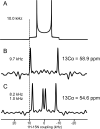

References
-
- Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff HW, Philips DC. Nature. 1958;181:666. - PubMed
-
- Perutz MF, Rossman MG, Cullis AF, Muirhead H, Will G, North AC. Nature. 1960;185:416–22. - PubMed
-
- Caffrey M. Ann Rev Biophys. 2009;38:29–51. - PubMed
-
- Sanders CR, Myers JK. Annu Rev Biophys Biomol Struct. 2004;33:25–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

