Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes
- PMID: 21964766
- PMCID: PMC3504975
- DOI: 10.1002/ijc.26471
Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes
Abstract
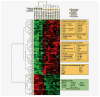
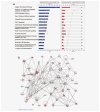
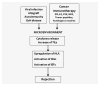
We present the results of a comparative gene expression analysis of 15 metastases (10 regressing and 5 progressing) obtained from 2 melanoma patients with mixed response following different forms of immunotherapy. Whole genome transcriptional analysis clearly indicate that regression of melanoma metastases is due to an acute immune rejection mediated by the upregulation of genes involved in antigen presentation and interferon mediated response (STAT-1/IRF-1) in all the regressing metastases from both patients. In contrast, progressing metastases showed low transcription levels of genes involved in these pathways. Histological analysis showed T cells and HLA-DR positive infiltrating cells in the regressing but not in the progressing metastases. Quantitative expression analysis of HLA-A,B and C genes on microdisected tumoral regions indicate higher HLA expression in regressing than in progressing metastases. The molecular signature obtained in melanoma rejection appeared to be similar to that observed in other forms of immune-mediated tissue-specific rejection such as allograft, pathogen clearance, graft versus host or autoimmune disease, supporting the immunological constant of rejection. We favor the idea that the major factor determining the success or failure of immunotherapy is the nature of HLA Class I alterations in tumor cells and not the type of immunotherapy used. If the molecular alteration is reversible by the immunotherapy, the HLA expression will be upregulated and the lesion will be recognized and rejected. In contrast, if the defect is structural the MHC Class I expression will remain unchanged and the lesion will progress.
Copyright © 2011 UICC.
Figures

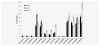
References
-
- Terando AM, Faries MB, Morton DL. Vaccine therapy for melanoma: current status and future directions. Vaccine. 2007;25:4–16. - PubMed
-
- Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, Gogas HJ. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–55. - PubMed
-
- Geldmacher A, Freier A, Losch FO, Walden P. Therapeutic vaccination for cancer immunotherapy: antigen selection and clinical responses. Hum Vaccine. 2011;7S:115–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

