Sleep disturbance in euthymic bipolar patients
- PMID: 21965189
- PMCID: PMC3787715
- DOI: 10.1177/0269881111421973
Sleep disturbance in euthymic bipolar patients
Abstract
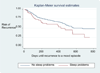
Sleep disturbance is a common feature during mood episodes in bipolar disorder. The aim of this study was to investigate the prevalence of such symptoms among euthymic bipolar patients, and their association with risk for mood episode recurrence. A cohort of bipolar I and II subjects participating in the Systematic Treatment Enhancement Program for Bipolar Disorder who were euthymic for at least 8 weeks were included in this analysis. Survival analysis was used to examine the association between sleep disturbance on the Montgomery-Asberg Depression Rating Scale (MADRS) and recurrence risk. A total of 73/483 bipolar I and II subjects reported at least mild sleep disturbance (MADRS sleep item ≥2) for the week prior to study entry. The presence of sleep problems was associated with a history of psychosis, number of previous suicide attempts, and anticonvulsant use. Sleep disturbance at study entry was significantly associated with risk for mood episode recurrence. Sleep disturbance is not uncommon between episodes for individuals with bipolar disorder and may be associated with a more severe course of illness. This suggests that sleep disturbance is an important prodromal symptom of bipolar disorder and should be considered a target for pharmacologic or psychosocial maintenance treatment.
Conflict of interest statement
Conflict of Interest Statement
Dr. Sylvia is a consultant for United BioSource Corporation.
Dr. Dupuy does not have any support or funding to disclose.
In the past two years, Dr. Ostacher has served on advisory/consulting boards of Pfizer and Schering Plough (now Merck); he has received speaking fees from AstraZeneca, Bristol Myers Squibb, Eli Lilly & Company, and Pfizer.
Ms. Cowperthwait does not have any support or funding to disclose.
Ms. Hay is a consultant for GENOMIND.
Over his lifetime, Dr. Sachs has served as a member of the speakers’ bureaus for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Eli Lilly & Co, GlaxoSmithKline, Janssen Pharmaceuticals, Memory Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Sanofi-Aventis, and Wyeth. Dr. Sachs has received research support from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Eli Lilly & Co, GlaxoSmithKline, Janssen Pharmaceuticals, Memory Pharmaceuticals, the National Institute of Mental Health, Novartis Pharmaceuticals, Pfizer, Repligen, Shire, and Wyeth. In his lifetime, Dr. Sachs has served on advisory/consulting boards of Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Cephalon, CNS Response, Elan Pharmaceuticals, Memory Pharmaceuticals, Merck, Novartis Pharmaceuticals, Organon, Otsuka, Pfizer, Schering Plough, Sepracor, Repligen, Sanofi-Aventis, Shire, Sigma-Tau, Solvay Pharmaceuticals, and Wyeth. Dr. Sachs and his spouse own stock in Concordant Rater Systems. In the past twelve months, Dr. Sachs has received research support from GlaxoSmithKline, the National Institute of Mental Health, and Repligen. In the past twelve months, Dr. Sachs has served on the advisory/consulting boards of AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Cephalon, Concordant Rater Systems, Janssen Pharmaceuticals, Merck, Otsuka, Pfizer, Schering Plough, Sepracor, and Repligen.
Dr. Nierenberg is a full time employee of the Massachusetts General Hospital (MGH). In the past 12 months (as of June 21, 2010), he has served as a consultant to the Appliance Computing Inc. (Mindsite), and Brandeis University. Through the MGH Clinical Trials Network and Institute (CTNI), he has consulted for Brain Cells, Inc, Dianippon Sumitomo/Sepracor, Labopharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck Pharmaceuticals. He received grant/research support through MGH from NIMH, PamLabs, Pfizer Pharmaceuticals, and Shire. He received honoraria from Belvior Publishing, Hillside Hospital, American Society for Clinical Psychopharmacology, Columbia University, IMEDEX, MJ Consulting, New York State, MBL Publishing, Physicians Postgraduate Press, SciMed, SUNY Buffalo, University of Wisconsin, and the University of Pisa. Dr. Nierenberg is a presenter for the Massachusetts General Hospital Psychiatry Academy (MGHPA). The education programs conducted by the MGHPA were supported through Independent Medical Education (IME) grants from the following pharmaceutical companies in 2008: Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals; in 2009 Astra Zeneca, Eli Lilly, and Bristol-Myers Squibb. No speaker bureaus or boards since 2003. He is on the advisory boards of Appliance Computing, Inc., Brain Cells, Inc., Eli Lilly and Company, and Takeda/Lundbeck and Targacept. Dr. Nierenberg owns stock options in Appliance Computing, Inc. and Brain Cells, Inc. Through MGH, he is named for copyrights to the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the CTNI.
Dr. Perlis receives speaker's bureau/consulting fees from AstraZeneca, Eli Lilly & Co., Proteus Biomedical, and Concordant Rater Systems. Dr. Perlis receives royalties and has a patent for the Concordant Rater Systems.
Figures
Similar articles
-
Circadian Rhythm Sleep-Wake Disorders Predict Shorter Time to Relapse of Mood Episodes in Euthymic Patients With Bipolar Disorder: A Prospective 48-Week Study.J Clin Psychiatry. 2018 Jan/Feb;79(1):17m11565. doi: 10.4088/JCP.17m11565. J Clin Psychiatry. 2018. PMID: 29286593
-
Cognitive behavioral therapy for insomnia in euthymic bipolar disorder: study protocol for a randomized controlled trial.Trials. 2014 Jan 16;15:24. doi: 10.1186/1745-6215-15-24. Trials. 2014. PMID: 24433249 Free PMC article.
-
Neurocognitive impairment and psychosis in bipolar I disorder during early remission from an acute episode of mood disturbance.J Clin Psychiatry. 2010 Feb;71(2):201-6. doi: 10.4088/JCP.08m04663yel. Epub 2009 Nov 17. J Clin Psychiatry. 2010. PMID: 19925749 Free PMC article.
-
Considerations in the pharmacotherapy of bipolar disorder during and after pregnancy.Curr Drug Saf. 2011 Nov 1;6(5):318-23. doi: 10.2174/157488611798918656. Curr Drug Saf. 2011. PMID: 22424539 Review.
-
Cognitive deficits in bipolar disorders: Implications for emotion.Clin Psychol Rev. 2018 Feb;59:126-136. doi: 10.1016/j.cpr.2017.11.006. Epub 2017 Nov 21. Clin Psychol Rev. 2018. PMID: 29195773 Free PMC article. Review.
Cited by
-
Sleep disturbance and cognitive deficits in bipolar disorder: toward an integrated examination of disorder maintenance and functional impairment.Clin Psychol Rev. 2013 Feb;33(1):33-44. doi: 10.1016/j.cpr.2012.10.001. Epub 2012 Oct 8. Clin Psychol Rev. 2013. PMID: 23123569 Free PMC article. Review.
-
The significance of sleep quality in euthymic bipolar patients from Nigeria.S Afr J Psychiatr. 2022 Feb 16;28:1739. doi: 10.4102/sajpsychiatry.v28i0.1739. eCollection 2022. S Afr J Psychiatr. 2022. PMID: 35281965 Free PMC article.
-
Cognitive deficits in bipolar disorder: from acute episode to remission.Eur Arch Psychiatry Clin Neurosci. 2016 Apr;266(3):225-37. doi: 10.1007/s00406-015-0657-2. Epub 2015 Nov 26. Eur Arch Psychiatry Clin Neurosci. 2016. PMID: 26611783
-
Relationships between Sleep Quality, Introspective Accuracy, and Confidence Differ among People with Schizophrenia, Schizoaffective Disorder, and Bipolar Disorder with Psychotic Features.Behav Sci (Basel). 2024 Feb 28;14(3):192. doi: 10.3390/bs14030192. Behav Sci (Basel). 2024. PMID: 38540495 Free PMC article.
-
Sleep characteristics in child and adolescent offspring of parents with bipolar disorder: a case control study.BMC Psychiatry. 2017 May 26;17(1):199. doi: 10.1186/s12888-017-1361-8. BMC Psychiatry. 2017. PMID: 28549429 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
-
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-Retest Reliability and Validity of the Pittsburgh Sleep Quality Index in Primary Insomnia. J Psychosom Res. 2002;53:737–740. - PubMed
-
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal Relation between Sleep and Mood Ni Patients with Bipolar Disorder. Bipolar Disord. 2006;8:160–167. - PubMed
-
- Benedetti F, Colombo C, Serretti A, Lorenzi C, Pontiggia A, Barbini B, et al. Antidepressant Effects of Light Therapy Combined with Sleep Deprivation Are Influenced by a Functional Polymorphism within the Promoter of the Serotonin Transporter Gene. Biol Psychiatry. 2003;54:687–692. - PubMed
-
- Bliss R, Weinberg J, Vieira V, Webster T. Detecting Confounding And Evaluating The 10% Rule Joint Statistical Meetings. Miami Beach, FL: 2011.