In vivo and in vitro pharmacological studies of methoxycarbonyl-carboetomidate
- PMID: 21965364
- PMCID: PMC3252484
- DOI: 10.1213/ANE.0b013e3182320559
In vivo and in vitro pharmacological studies of methoxycarbonyl-carboetomidate
Abstract
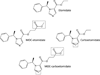
Background: We previously developed 2 etomidate analogs that retain etomidate's favorable hemodynamic properties but whose adrenocortical effects are reduced in duration or magnitude. Methoxycarbonyl (MOC)-etomidate is rapidly metabolized and ultrashort acting whereas (R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (carboetomidate) does not potently inhibit 11β-hydroxylase. We hypothesized that MOC-etomidate's labile ester could be incorporated into carboetomidate to produce a new agent that possesses favorable properties individually found in each agent. We describe the synthesis and pharmacology of MOC-(R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (MOC-carboetomidate), a "soft" analog of carboetomidate.
Methods: MOC-carboetomidate's octanol:water partition coefficient was determined chromatographically and compared with those of etomidate, carboetomidate, and MOC-etomidate. MOC-carboetomidate's 50% effective concentration (EC(50)) and 50% effective dose for loss of righting reflexes (LORR) were measured in tadpoles and rats, respectively. Its effect on γ-aminobutyric acid A (GABA(A)) receptor function was assessed using 2-microelectrode voltage clamp electrophysiological techniques and its metabolic stability was determined in pooled rat blood using high performance liquid chromatography. Its duration of action and effects on arterial blood pressure and adrenocortical function were assessed in rats.
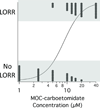
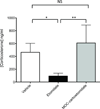
Results: MOC-carboetomidate's octanol:water partition coefficient was 3300 ± 280, whereas those for etomidate, carboetomidate, and MOC-etomidate were 800 ± 180, 15,000 ± 3700, and 190 ± 25, respectively. MOC-carboetomidate's EC(50) for LORR in tadpoles was 9 ± 1 μM and its EC(50) for LORR in rats was 13 ± 5 mg/kg. At 13 μM, MOC-carboetomidate enhanced GABA(A) receptor currents by 400% ± 100%. Its metabolic half-life in pooled rat blood was 1.3 min. The slope of a plot of the duration of LORR in rats versus the logarithm of the hypnotic dose was significantly shallower for MOC-carboetomidate than for carboetomidate (4 ± 1 vs 15 ± 3, respectively; P = 0.0004123). At hypnotic doses, the effects of MOC-carboetomidate on arterial blood pressure and adrenocortical function were not significantly different from those of vehicle alone.
Conclusions: MOC-carboetomidate is a GABA(A) receptor modulator with potent hypnotic activity that is more rapidly metabolized and cleared from the brain than carboetomidate, maintains hemodynamic stability similar to carboetomidate, and does not suppress adrenocortical function.
Conflict of interest statement
Name: Ervin Pejo, B.S.
Contribution: EP led in conduct of study and manuscript preparation. He attests to the integrity of the data and analysis.
Conflicts: EP has no conflicts of interest to declare.
Name: Joseph F. Cotten M.D., Ph.D.
Contribution: JFC assisted in study design, data interpretation, and manuscript preparation.
Conflicts: JFC is a co-inventor on a patent application submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-carboetomidate or related analogs.
Name: Elizabeth W. Kelly
Contribution: EWK performed the electrophysiology experiments
Conflicts: EWK has no conflicts of interest to declare.
Name: Ri Le Ge, M.D., Ph.D.
Contribution: RLG assisted in study conduct.
Conflicts: RLG has no conflicts of interest to declare.
Name: Gregory D. Cuny, Ph.D.
Contribution: GDC designed the synthetic pathways for the syntheses of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: GDC is a co-inventor on a patent application submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-carboetomidate or related analogs.
Name: Joydev K. Laha Ph.D.
Contribution: JKL help to design the synthetic pathways for the syntheses of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: JKL has no conflicts of interest to declare.
Name: Jifeng Liu, Ph.D.
Contribution: JL oversaw the synthesis and purification of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: JL has no conflicts of interest to declare.
Name: Xiang Jie Lin, MSc
Contribution: XJL performed the synthesis and purification of methoxycarbonyl-carboetomidate and carboetomidate.
Conflicts: XJL has no conflicts of interest to declare.
Name: Douglas E. Raines, M.D.
Contribution: DER conceived of methoxycarbonyl-carboetomidate, assisted in study design, data interpretation, and manuscript preparation.
Attestation: Dr. Raines attests to the integrity of the data and analysis.
Conflicts: DER is a co-inventor on a patent application submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-carboetomidate or related analogs. DER holds an equity position in Annovation BioPharma, a pharmaceutical company that seeks to develop technologies covered by that patent.
Figures





Similar articles
-
Carboetomidate: a pyrrole analog of etomidate designed not to suppress adrenocortical function.Anesthesiology. 2010 Mar;112(3):637-44. doi: 10.1097/ALN.0b013e3181cf40ed. Anesthesiology. 2010. PMID: 20179500 Free PMC article.
-
Pharmacological studies of methoxycarbonyl etomidate's carboxylic acid metabolite.Anesth Analg. 2012 Aug;115(2):305-8. doi: 10.1213/ANE.0b013e318239c6ca. Epub 2011 Nov 3. Anesth Analg. 2012. PMID: 22052979 Free PMC article.
-
Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression.Anesthesiology. 2009 Aug;111(2):240-9. doi: 10.1097/ALN.0b013e3181ae63d1. Anesthesiology. 2009. PMID: 19625798 Free PMC article.
-
The Role of GABA Receptors in Anesthesia and Sedation: An Updated Review.CNS Drugs. 2025 Jan;39(1):39-54. doi: 10.1007/s40263-024-01128-6. Epub 2024 Oct 27. CNS Drugs. 2025. PMID: 39465449 Free PMC article. Review.
-
Novel etomidate derivatives.Curr Pharm Des. 2012;18(38):6253-6. doi: 10.2174/138161212803832362. Curr Pharm Des. 2012. PMID: 22762475 Review.
Cited by
-
Electroencephalographic and hypnotic recoveries after brief and prolonged infusions of etomidate and optimized soft etomidate analogs.Anesthesiology. 2012 Nov;117(5):1037-43. doi: 10.1097/ALN.0b013e31826d3de2. Anesthesiology. 2012. PMID: 22929726 Free PMC article.
-
γ-Aminobutyric Acid Type A Receptor Modulation by Etomidate Analogs.Anesthesiology. 2016 Mar;124(3):651-63. doi: 10.1097/ALN.0000000000000992. Anesthesiology. 2016. PMID: 26691905 Free PMC article.
-
Competitive Antagonism of Anesthetic Action at the γ-Aminobutyric Acid Type A Receptor by a Novel Etomidate Analog with Low Intrinsic Efficacy.Anesthesiology. 2017 Nov;127(5):824-837. doi: 10.1097/ALN.0000000000001840. Anesthesiology. 2017. PMID: 28857763 Free PMC article.
-
Distinct Hypnotic Recoveries After Infusions of Methoxycarbonyl Etomidate and Cyclopropyl Methoxycarbonyl Metomidate: The Role of the Metabolite.Anesth Analg. 2016 Apr;122(4):1008-14. doi: 10.1213/ANE.0000000000001146. Anesth Analg. 2016. PMID: 26991617 Free PMC article.
-
Carboetomidate inhibits alpha4/beta2 neuronal nicotinic acetylcholine receptors at concentrations affecting animals.Anesth Analg. 2012 Jul;115(1):70-2. doi: 10.1213/ANE.0b013e318254273e. Epub 2012 Apr 27. Anesth Analg. 2012. PMID: 22543065 Free PMC article.
References
-
- Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM. Etomidate, R-(+)-ethyl-1-(-methyl-benzyl)imidazole-5-carboxylate (R 16659), a potent, short-acting and relatively atoxic intravenous hypnotic agent in rats. Arzneimittelforschung. 1971;21:1234–1243. - PubMed
-
- Vinson DR, Bradbury DR. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med. 2002;39:592–598. - PubMed
-
- Guldner G, Schultz J, Sexton P, Fortner C, Richmond M. Etomidate for rapid-sequence intubation in young children: hemodynamic effects and adverse events. Acad Emerg Med. 2003;10:134–139. - PubMed
-
- Zed PJ, Abu-Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Acad Emerg Med. 2006;13:378–383. - PubMed
-
- Dhawan N, Chauhan S, Kothari SS, Kiran U, Das S, Makhija N. Hemodynamic responses to etomidate in pediatric patients with congenital cardiac shunt lesions. J Cardiothorac Vasc Anesth. 2010;24:802–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

