Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions
- PMID: 21965410
- PMCID: PMC3233039
- DOI: 10.1128/AEM.05382-11
Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions
Abstract
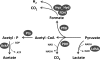
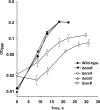
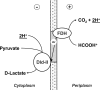
Shewanella oneidensis MR-1 is a facultative anaerobe that derives energy by coupling organic matter oxidation to the reduction of a wide range of electron acceptors. Here, we quantitatively assessed the lactate and pyruvate metabolism of MR-1 under three distinct conditions: electron acceptor-limited growth on lactate with O(2), lactate with fumarate, and pyruvate fermentation. The latter does not support growth but provides energy for cell survival. Using physiological and genetic approaches combined with flux balance analysis, we showed that the proportion of ATP produced by substrate-level phosphorylation varied from 33% to 72.5% of that needed for growth depending on the electron acceptor nature and availability. While being indispensable for growth, the respiration of fumarate does not contribute significantly to ATP generation and likely serves to remove formate, a product of pyruvate formate-lyase-catalyzed pyruvate disproportionation. Under both tested respiratory conditions, S. oneidensis MR-1 carried out incomplete substrate oxidation, whereby the tricarboxylic acid (TCA) cycle did not contribute significantly. Pyruvate dehydrogenase was not involved in lactate metabolism under conditions of O(2) limitation but was required for anaerobic growth, likely by supplying reducing equivalents for biosynthesis. The results suggest that pyruvate fermentation by S. oneidensis MR-1 cells represents a combination of substrate-level phosphorylation and respiration, where pyruvate serves as an electron donor and an electron acceptor. Pyruvate reduction to lactate at the expense of formate oxidation is catalyzed by a recently described new type of oxidative NAD(P)H-independent d-lactate dehydrogenase (Dld-II). The results further indicate that pyruvate reduction coupled to formate oxidation may be accompanied by the generation of proton motive force.
Figures
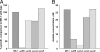
References
-
- Bowman J. P., et al. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5 omega 3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 47: 1040–1047 - PubMed
-
- Brown T. D. K., Jones-Mortimer M. C., Kornberg H. L. 1977. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J. Gen. Microbiol. 102: 327–336 - PubMed
-
- Champine J. E., Underhill B., Johnston J. M., Lilly W. W., Goodwin S. 2000. Electron transfer in the dissimilatory iron-reducing bacterium Geobacter metallireducens. Anaerobe 6: 187–196
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

